GORE® EXCLUDER® AAA Endoprosthesis
Allows for confident endovascular repair of abdominal aortic aneurysms, with the unique ability to reconstrain the proximal end of the device and reposition it for ideal placement.
25+ years of advancing patient care: One man shares how the continued innovation of the EXCLUDER® Device family made a difference in his life.

25+ years of supporting post-trial follow-up: Dr. Ross Milner discusses why continued studies are helping advance aortic treatment.

25+ years of peer-reviewed publications: how one registry continues to contribute EVAR insights through literature.
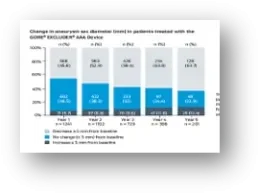
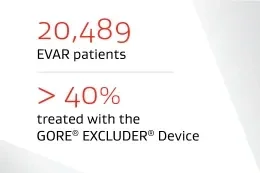
* Based on the number of Trunk-Ipsilateral Legs distributed.
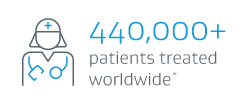
† Based on company-sponsored trials and registries shown on clinicaltrials.gov for currently available stent grafts.
Learn more about our family of EVAR solutions you can count on:
GORE® EXCLUDER® Iliac Branch Endoprosthesis
GORE® EXCLUDER® Conformable AAA Endoprosthesis
Featured resources

GORE® EXCLUDER® AAA Endoprosthesis
INDICATIONS FOR USE IN THE U.S.: Trunk-Ipsilateral Leg and Contralateral Leg Endoprosthesis. The GORE® EXCLUDER® AAA Endoprosthesis is intended to exclude the aneurysm from the blood circulation in patients diagnosed with infrarenal abdominal aortic aneurysm (AAA) disease and who have appropriate anatomy as described below: Adequate iliac/femoral access; Infrarenal aortic neck treatment diameter range of 19–32 mm and a minimum aortic neck length of 15 mm; Proximal aortic neck angulation ≤ 60°; Iliac artery treatment diameter range of 8–25 mm and iliac distal vessel seal zone length of at least 10 mm. Aortic Extender and Iliac Extender Endoprosthesis. The Aortic and Iliac Extender Endoprostheses are intended to be used after deployment of the GORE® EXCLUDER® AAA Endoprosthesis. These extensions are intended to be used when additional length and/or sealing for aneurysmal exclusion is desired. CONTRAINDICATIONS: The GORE® EXCLUDER® AAA Endoprosthesis is contraindicated in: patients with known sensitivities or allergies to the device materials; patients with a systemic infection who may be at increased risk of endovascular graft infection. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231241802-EN