CBAS® Heparin Surface
Proven heparin bonding technology for lasting thromboresistance
Dedicated to Innovation: Thromboresistance in Vascular Devices
Gore’s CBAS® Heparin Surface, the proven heparin bonding technology for lasting thromboresistance, is used in many of our interventional and vascular surgery products. End-point covalent bonding keeps heparin anchored to the device, while the bioactive site remains free to interact with the blood to help prevent clotting.*
- Proven heparin availability: Performance-ready heparin active site*,1,4
- Proven heparin bioactivity: Unmatched, persistent ability to take up antithrombin*,2,3
- Proven lasting thromboresistance: Improved surface hemocompatibility resulting from heparin availability and bioactivity*,1-5
Proprietary covalent end-point bonding
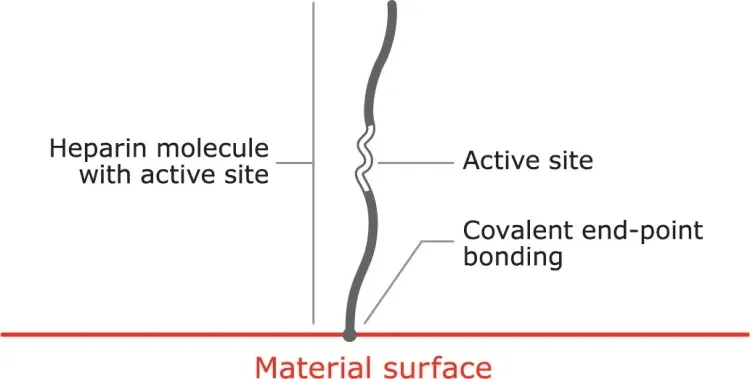
Covalent end-point bonding allows the heparin to extend into the bloodstream, keeping the active site bioavailable, unlike a non-permanent bond that can be washed away in the bloodstream.
- The anticoagulant function of heparin is dependent on the bioavailability of an active site within the molecule.
- Some methods of covalent heparin bonding damage and / or obstruct the active site, and hence destroy heparin’s anticoagulant activity.
- The CBAS® Heparin Surface consists of a proprietary covalent end-point bond that preserves the active site, thus retaining heparin’s anticoagulant activity.
Mechanism of action
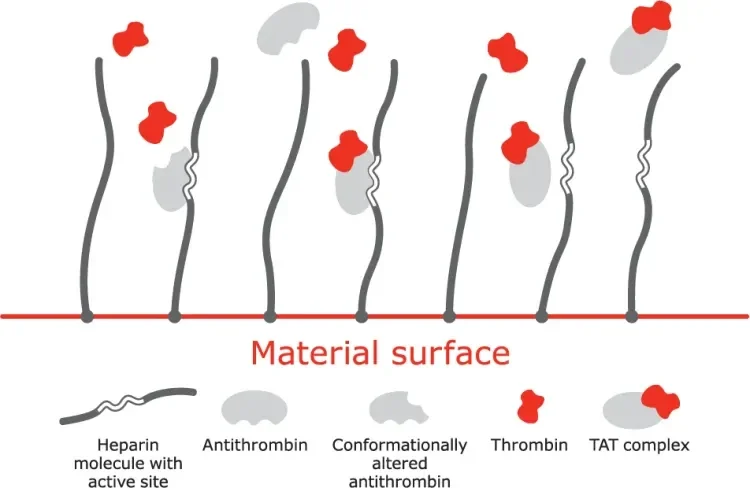
A. Bioactive site of the heparin molecule enables antithrombin to bind thrombin.
B. When antithrombin binds to thrombin, a neutral thrombin antithrombin (TAT) complex is formed.
C. Neutral TAT complex detaches from the heparin molecule. Active site becomes available to again bind antithrombin.
* See full CBAS® Heparin Surface references.
CBAS® is a trademark of Carmeda AB, a wholly owned subsidiary of W. L. Gore & Associates, Inc.
Featured Gore Products with CBAS® Heparin Surface
GORE® ACUSEAL Vascular Graft
GORE® PROPATEN® Vascular Graft
GORE® PROPATEN® Vascular Graft configured for Pediatric Shunt
GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
22589259-EN