GORE® PROPATEN® Vascular Graft
Addressing the problem of thrombus formation on the luminal surface of a vascular graft is a challenge faced by all vascular surgeons. The GORE® PROPATEN® Vascular Graft is specifically designed for those vascular procedures in which the risk of acute graft thrombotic failure is of clinical concern.
Anything better below-knee would be a vein
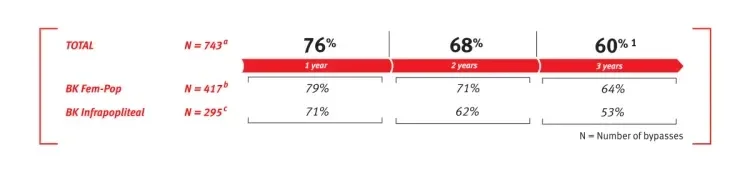
Overall weighted average* primary patency based on GORE® PROPATEN® Vascular Graft literature in below knee bypasses
*See References (PDF)
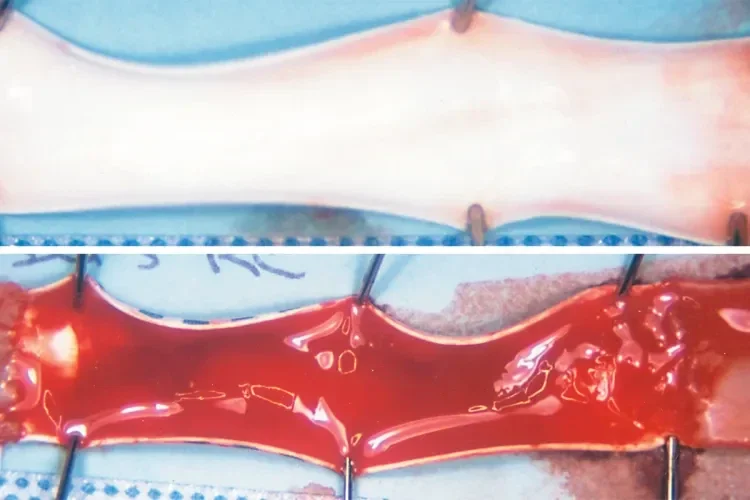
The BioActive luminal surface of a 3 mm diameter GORE® PROPATEN® Vascular Graft (top) remains free of thrombus, while the non-bioactive surface of a control graft (below; 3 mm diameter) is covered with thrombus. Grafts were explanted after 2 hours in a challenging carotid shunt canine model.
The GORE® PROPATEN® Vascular Graft harnesses the anticoagulant properties of heparin directly at the luminal surface of the graft. The proprietary end-point attachment mechanism, the CBAS Heparin Surface, serves to anchor heparin molecules to the luminal surface while still maintaining heparin's intrinsic bioactive properties. The result: a thromboresistant bioactive graft surface that retains its bioactive properties.1
The GORE® PROPATEN® Vascular Graft has demonstrated improved thromboresistance and patency compared to standard ePTFE grafts in pre-clinical in vivo tests. A randomized, multi-center clinical study has shown promising results.2 The thromboresistant surface technology employed on the GORE® PROPATEN® Vascular Graft is designed to maintain bioactivity, thus increasing the potential for performance improvement and increased patency*.
*Long-term data are not available regarding improved patency compared to marketed grafts.
-
Begovac PC, Thomson RC, Fisher JL, Hughson A, Gällhagen A. Improvements in GORE-TEX® Vascular Graft performance by Carmeda® bioactive surface heparin immobilization.European Journal of Vascular & Endovascular Surgery 2003;25(5):432-437.
Lindholt JS, Gottschalksen B, Johannesen N, et al. The Scandinavian Propaten® Trial – 1-year patency of PTFE vascular prostheses with heparin-bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses – a randomised clinical controlled multi-centre trial. European Journal of Vascular & Endovascular Surgery 2011;41(5):668-673.
Featured resources
Related to this product

A Decade of Performance
Visit propatenperformance.com to learn more.