GORE® SYNECOR Biomaterial
Strong, permanent mesh with a low infection rate for high-BMI or co-morbid patients.1,2


2.5 x
stronger than BD® BARD® Soft Mesh*,3,4
14%
Stronger than BD® VENTRALIGHT® ST Mesh*,4,5
ZERO
fractures reported†,‡
Permanent mesh solution for ventral hernia repair with the strength of a heavyweight, built like a lightweight and a low rate of infection for high BMI or co-morbid patients.1-7 All these benefits are realized at a lower cost than BD® VENTRALIGHT® ST Mesh.†,§
STRENGTH
Permanent devices
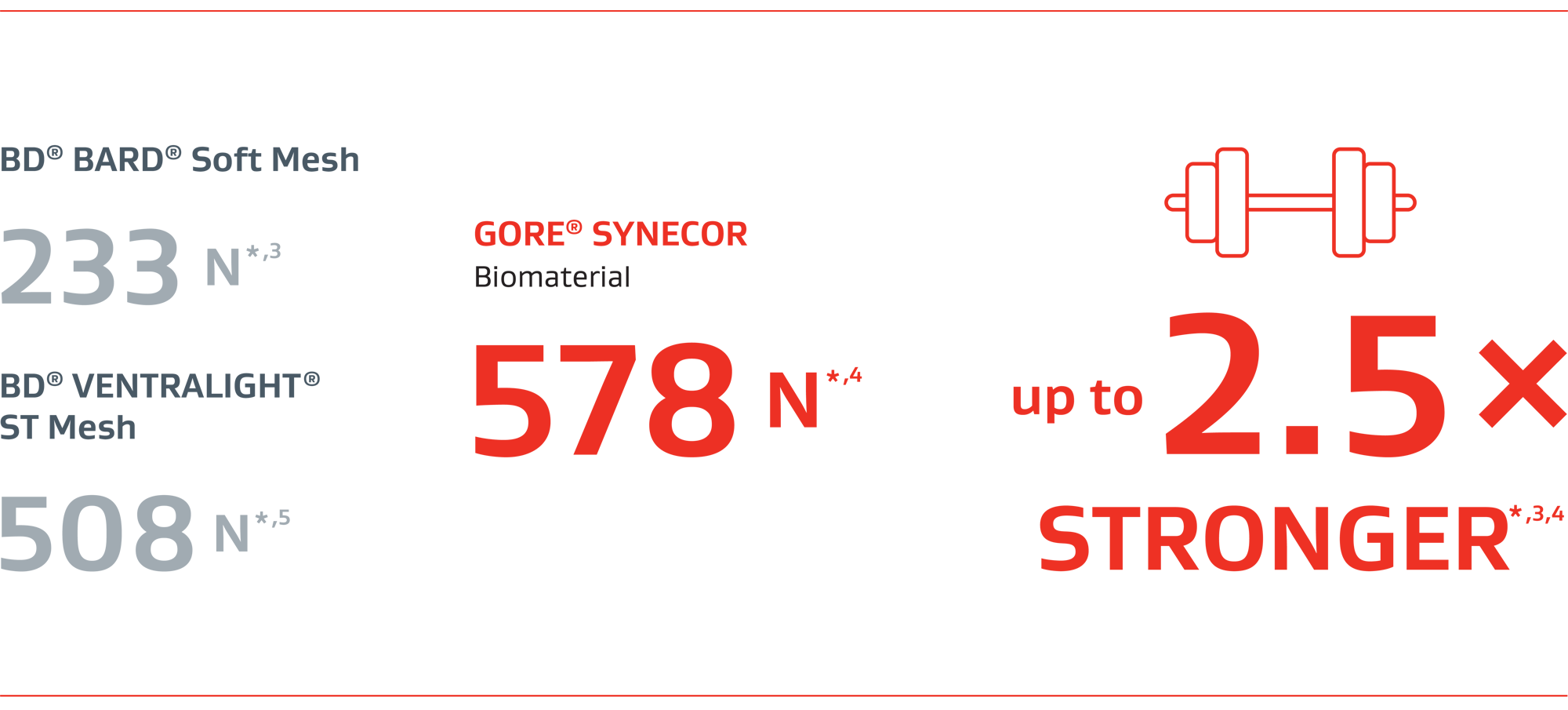
Ball burst strengthII (N)
GORE® SYNECOR Biomaterial
< 1% recurrence rate in ventral hernia working group (VHWG) 1 and 2 patients1,2,8
≤ 0.7% device-related adverse event rate through 12 months1,8
Easy to use in minimally invasive procedures (laparoscopic, robotic) and open surgeries6,7
See the real-world evidence: Evaluation of long-term performance of an intraperitoneal biomaterial in the treatment of ventral hernias—Surgical Endoscopy December, 2023
Gore ventral hernia solutions balance strength with a low rate of complications for your high-risk patients which is backed by†,¶:
- 15 years of extensive clinical literature in soft tissue reinforcement†
- Over 200 publications††
- More than 5,000 cases in literature††

See it in action
* Includes only the permanent component of these products.
† Data on file, W. L. Gore & Associates, Inc; Flagstaff, AZ.
‡ No confirmed reports of fracture.
§ Cost savings are based on 90 days post-operative outcomes.
II Bench-top evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results.
¶ GORE® BIO-A® Tissue Reinforcement, GORE® ENFORM Biomaterial and GORE® SYNECOR Biomaterial.
** No confirmed or published complete mesh removals due to infection or erosions reported.
†† A literature search was performed by an Information Specialist in 2024 using the DIMENSIONS® Database, EMBASE® Database, and MEDLINE® on DIALOG® Database. Key words/phrases are on file.
‡‡ Largest range of sizes currently available in a permanent heavyweight soft tissue reinforcement device in the United States as of July 2024.
§§ Out-of-the-box strength.
- Linn JG, Mallico EJ, Doerhoff CR, Grantham DW, Washington RG Jr. Evaluation of long-term performance of an intraperitoneal biomaterial in the treatment of ventral hernias. Surgical Endoscopy 2023;37:3455-3462.
- Rios-Diaz A, Hitchner M, Christopher AN, Broach R, Cunning JR, Fischer JP. Early clinical and patient-reported outcomes of a new hybrid mesh for incisional hernia repair. Journal of Surgical Research 2021;265:49-59.
- Olson TB. Competitive Hernia Device Strength Evaluation. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2016. [Work plan]. WP108484.
- W. L. Gore & Associates, Inc. Plexus Knit PQ Validation Report. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2022. [Validation Report]. MD145325. Rev 5.
- Olson TB. Ventralight ST Strength after 14 and 28 day Degradation of Absorbable Components. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2016. [Work plan]. WP108781.
- W. L. Gore & Associates, Inc. GORE SYNECOR Intraperitoneal Biomaterial Design Control (DC) Matrix. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2023. [Design Control Matrix – DC Matrix]. MD184278. Rev 3.
- W. L. Gore & Associates, Inc. GORE SYNECOR Preperitoneal Biomaterial Design Control (DC) Matrix. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2024. [Design Control Matrix – DC Matrix]. MD187124. Rev 4.
- Goldblatt MI, Reynolds M, Doerhoff CR, et al. Ventral hernia repair with a hybrid absorbable-permanent preperitoneal mesh. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. In press.
- Deeken CR, Matthews BD. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-PHASIX Mesh) in a porcine model of hernia repair. ISRN Surgery 2013;2013:238067.
BD, BARD, PHASIX and VENTRALIGHT are trademarks of C.R. Bard, Inc.
DIMENSIONS is a trademark of Digital Science & Research Solutions Inc.
EMBASE is a trademark of Elsevier Limited.
DIALOG is a trademark of Proquest LLC.
MEDLINE is a trademark of The National Library of Medicine.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE: The GORE® SYNECOR Intraperitoneal Biomaterial device is intended for use in the repair of hernias and abdominal wall or thoracic wall soft tissue deficiencies that may require the addition of a nonabsorbable reinforcing or bridging material.
CONTRAINDICATIONS: The GORE® SYNECOR Intraperitoneal Biomaterial is contraindicated for use in reconstruction of cardiovascular defects.
INDICATIONS FOR USE: The GORE® SYNECOR Preperitoneal Biomaterial device is intended for use in the repair of hernias and abdominal wall soft tissue deficiencies that may require the addition of a non-absorbable reinforcing or bridging material.
CONTRAINDICATIONS: The GORE® SYNECOR Preperitoneal Biomaterial is contraindicated for use in reconstruction of cardiovascular defects.
GORE® SYNECOR Biomaterial is not authorized for use in Canada.


