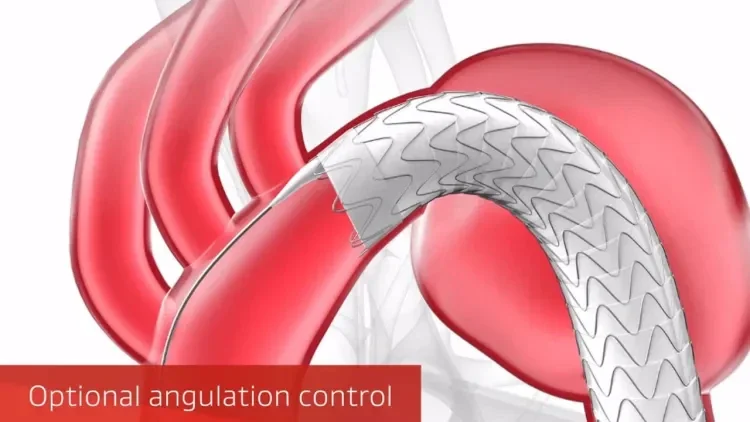
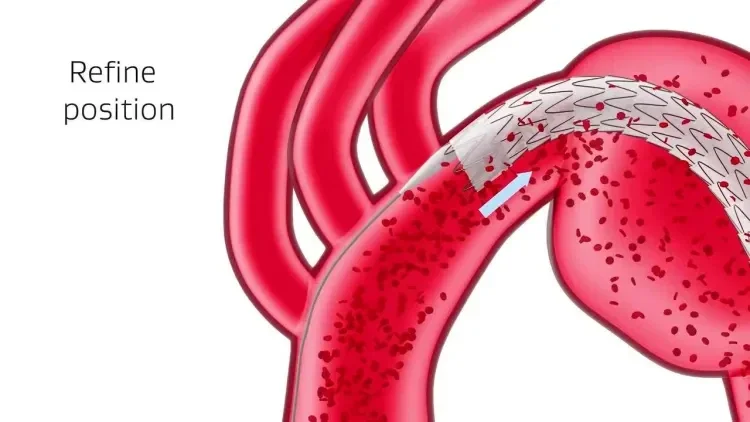
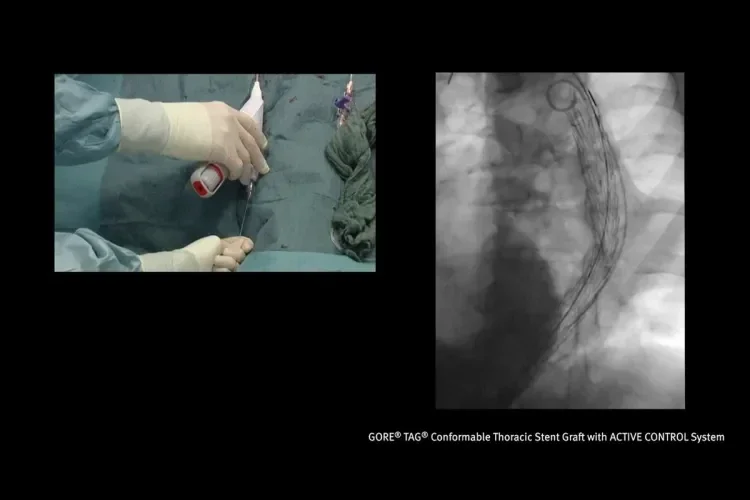
Designed for the demands of TEVAR
Six key considerations to help you deliver optimal results.
Hemodynamic stability

Unique oversizing window

Conformability
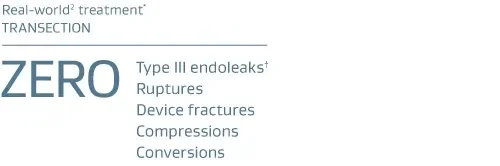
* GORE® TAG® Conformable Device.
† Endoleak requiring intervention. No endoleaks persisted past one month or required intervention.
- W. L. Gore & Associates. Observational Registry Characterizing the Performance and Feature Use of the GORE® TAG® Conformable Thoracic Stent Graft Featuring ACTIVE CONTROL System. NLM Identifier: NCT03286400. Published September 18, 2017. Updated December 17, 2020. Accessed February 23, 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT03286400
- GREAT is a prospective, observational, multicenter registry to actively track Gore commercial aortic endovascular device performance and associated patient outcomes in global markets with 10 years of follow-up. Data June 2017. Through 2-year follow-up. Transection n = 53.
241345600-EN