What if procurement could be a driver of sustainability and patient outcomes at the same time?
Innovating for a sustainable future
At Gore, we put solutions that improve life at the heart of our innovation. Our commitment to making a positive impact drives us to optimize our operations, reduce carbon emissions and minimize waste. As a global company, we are actively engaged in 45 projects focused on environmental sustainability. We understand that building a sustainable future is a continuous journey. Why not join us on this journey?



Committed to carbon reduction
We are dedicated to significantly reducing our carbon footprint. By 2025, our goal is to cut our scope 1 & 2† carbon emissions – those directly linked to our operations and energy use – by 50%. This means reducing emissions from sources such as fossil fuels used on-site and outsourced electricity and heating.
To achieve this, we have adopted a science-driven approach, aligning our efforts with the GHG Protocol Corporate Standard‡ to ensure transparent and accurate tracking of our greenhouse gas emissions. This is more than a goal; it's a crucial step towards a more sustainable future.
Enterprise Sustainability Update 2023
Durable performance for a more sustainable hospital
Sustainable practices in the operating room (OR) go beyond just reducing costs – they are about significantly improving the environmental impact. Beyond essential efforts to minimize medical waste, implement energy saving strategies and cut gas emissions, an effective intervention is often the one that does not take place at all.

Our products are meticulously designed for lasting performance, that may lead to shorter hospital stays and fewer complications possibly requiring reinterventions.1-11 This efficiency may help save hospital resources while simultaneously reducing waste and lowering the carbon footprint, making health care both more economical and more environmentally friendly.

From paper to pixels: Saving 70,000 kg annually with eIFUs
Instructions for Use (IFU) are essential for the safe and effective use of medical devices, guiding both health care professionals and patients. As printed Instructions for Use result in the use of large volumes of paper, we are in the process of becoming "paperless" by transitioning to electronic IFUs (eIFUs) by early 2025.§ This shift will save approximately 70,000 kg of paper annually, supporting our commitment to sustainability.
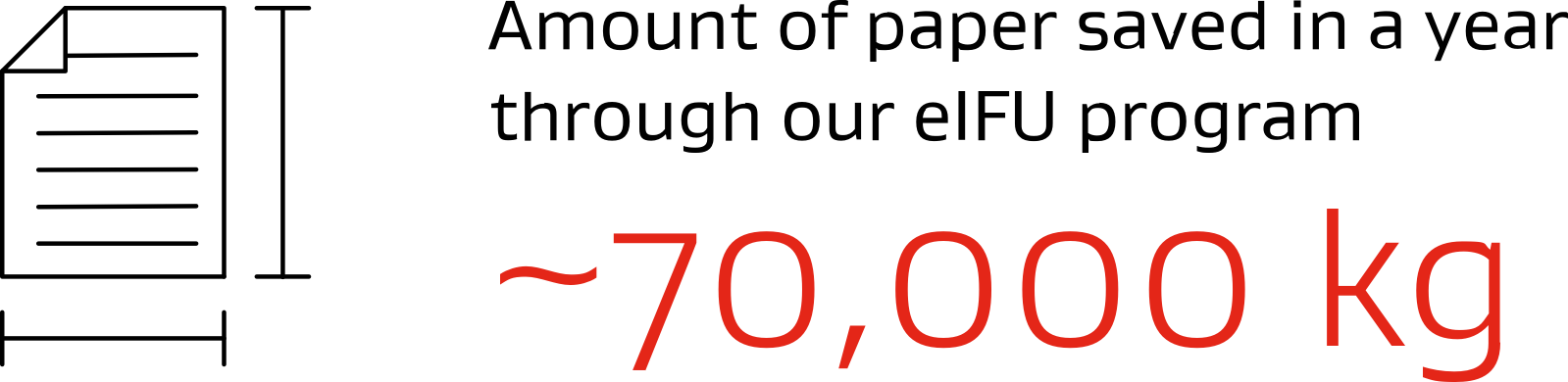
Thoughtful packaging for a reduced environmental impact
Gore has redesigned its medical packaging systems to minimize material use and reduce waste. By eliminating PVCII, optimizing the use of shippers and selecting FSC-certified¶ paper products, we aim to lessen our environmental footprint. Additionally, we are selectively converting existing packaging designs from a double sterile barrier to a single sterile barrier to reduce packaging material waste, while maintaining safety, quality and regulatory requirements.**
Enhancing environmental footprint evaluation with life cycle assessment
Life cycle assessment (LCA)†† is a widely recognized scientific method for evaluating the environmental impact of our products, such as their carbon footprint, waste production and water usage. It helps us find ways to reduce these impacts throughout the product’s entire value chain. At Gore we are actively pursuing LCA of devices in our portfolio.
These results inform product design and manufacturing improvements, helping us and our customers set meaningful sustainability key performance indicators (KPIs). We regard LCA as an ongoing, interdisciplinary learning process that drives continuous improvement.
High-quality vascular, cardiac, general surgery devices
Our portfolio of vascular, cardiac and general surgery medical devices is designed to help physicians improve patient outcomes.
Contact us to learn more about what we can do for you as a health care provider
* compared to baseline year 2016
† Scope 1 emissions are direct emissions from fossil fuel used at the plants and company vehicles; scope 2 are indirect emissions from purchased electricity, steam, heating and cooling.
‡ https://ghgprotocol.org/sites/default/files/standards/ghg-protocol-revised.pdf
§ Gore accessories will continue with a paper IFU for now. Gore is actively converting products by region to electronic instructions for use (IFU) to reduce paper waste associated with paper IFUs.
II PVC: polyvinyl chloride; a synthetic thermoplastic material made by polymerizing vinyl chloride. The properties depend on the added plasticizer.
¶ The Forest Stewardship Council (FSC) certifies forests to ensure their environments are responsibly managed and meet the highest environmental and social standards.
** Completed for STENT GRAFT TRUNK IPSILATERAL LEG C3 component of GORE® EXCLUDER® AAA Endoprosthesis and also Reduced Profile GORE® VIABAHN® VBX® Endoprosthesis
†† (LCA) Commonly used environmental accounting frameworks such as Carbon Footprinting, Environmental Product Declarations (EPD) and Product Environmental Footprints (PEF), as well as sector-specific frameworks such as the Higg Index for Fabrics, originate from and adhere to the methodological foundations of an LCA.
1. Bureau C, Thabut D, Oberti D, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152(1):157-163.
2. Iqbal K, Schumann E. Cost-consequence analysis of EPTFE vascular grafts with heparin end point covalent bond compared to standard EPTFE vascular grafts in below-knee surgical bypass for critical limb ischemia pad patients in Germany. Presented at ISPOR Europe (International Society for Pharmacoeconomics and Outcomes); November 6-9, 2022; Vienna, Austria. Value in Health 2022;25(12)Supplement:S114. EE306.
3. Vergnaud S, Riche VP, Tessier P, Mauduit N, Kaladji A, Gouëffic Y. Budget impact analysis of heparin-bonded polytetrafluoroethylene grafts (Propaten) against standard polytetrafluoroethylene grafts for below-the-knee bypass in patients with critical limb ischaemia in France. BMJ Open 2018;8(2):e017320.
4. Raithel D, Qu L, Hetzel G. Optimal EVAR fixation: infrarenal fixation is the safes option. Endovascular Today 2005;4(8):62-65
5. Stokmans RA, Teijink JA, Forbes TL, et al. Early results from the ENGAGE registry: real-world performance of the Endurant Stent Graft for endovascular AAA repair in 1262 patients. European Journal of Vascular & Endovascular Surgery 2012;44(4):369-375.
6. Hernández-Gea V, Procopet B, Giráldez Á, et al; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS improves outcome in high-risk variceal bleeding: an observational study. Hepatology 2019;69(1):282-293.
7. Canaud L, Gandet T, Sfeir J, Ozdemir BA, Solovei L, Alric P. Risk factors for distal stent graft-induced new entry tear after endovascular repair of thoracic aortic dissection. Journal of Vascular Surgery 2019;69(5):1610-1614.
8. Tjaden BL Jr, Sandhu H, Miller C, et al. Outcomes from the Gore Global Registry for Endovascular Aortic Treatment in patients undergoing thoracic endovascular aortic repair for type B dissection. Journal of Vascular Surgery 2018;68(5):1314-1323.
9. Vergnaud S, Riche VP, Tessier P, Mauduit N, Kaladji A, Gouëffic Y. Budget impact analysis of heparin-bonded polytetrafluoroethylene grafts (Propaten) against standard polytetrafluoroethylene grafts for below-the-knee bypass in patients with critical limb ischaemia in France. BMJ Open 2018;8(2):e017320
10. Gouëffic Y, Favre JP, Steinmetz E, et al. A randomized controlled trial comparing crude versus heparin-bonded PRFE graft in below the knee bypass surgery for critical limb ischemia (REPLACE trial). Design and protocol. Annals of Vascular Surgery 2019;58:115-121.
11. O’Neill F, Nakum M, Alerta H, Ankeny R, Wlatham M. A comparative economic analysis of the GORE® EXCLUDER® AAA Endoprosthesis versus competitive grafts: real world data from two prospective observational multicentre registries. – Presented at the Vascular Societies’ Annual Scientific Meeting; November 24-27, 2020 ; Virtual. O60.
12. Squiers JJ, DiMaio JM, Schaffer JM, et al. Surgical debranching versus branched endografting in zone 2 thoracic endovascular aortic repair. Journal of Vascular Surgery 2022;75(6):1829-1836.e3.
13. Czerny M, Schmidli J, Adler S, et al. Editor's Choice - Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an Expert Consensus Document of the European Association for Cardio-Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). European Journal of Vascular & Endovascular Surgery 2019;57(2):165-198.
14. Rizvi AZ, Murad MH, Fairman RM, Erwin PJ, Montori VM. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. Journal of Vascular Surgery 2009;50(5):1159-1169.
15. Chen X, Wang J, Premaratne S, Zhao J, Zhang WW. Meta-analysis of the outcomes of revascularization after intentional coverage of the left subclavian artery for thoracic endovascular aortic repair. Journal of Vascular Surgery 2019;70(4):1330-1340.
![]()
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
24PR2042-EN01