What if procurement could also impact patient outcomes?
Gore information for health care providers that are involved in the purchasing of medical devices
High-quality vascular, cardiac, general surgery devices
Health care providers face the challenge of delivering high-quality care and improving patient outcomes while reducing costs and becoming more efficient. The pressure on purchasing organizations is now more significant than ever before in the wake of the pandemic.
As a materials science company, Gore has a legacy built on innovation, developing high-quality products that improve patients' lives. But at Gore, we are about more than just products designed to improve patient outcomes. We believe in working in partnership with purchasers and the medical team. Our value-based services help both purchasers and physicians achieve medical and economic objectives.
Medical devices designed to improve patient outcomes
Our medical devices are designed to help physicians improve patient outcomes. The GORE® EXCLUDER® Conformable AAA Endoprosthesis with ACTIVE CONTROL System is just one example: It is the only device with angulation control. It provides a lower-risk alternative to open surgery, offering the benefits of less invasive repair to more abdominal aortic aneurysm (AAA) patients.1
Designed to exclude aneurysm from circulation and avoid rupture
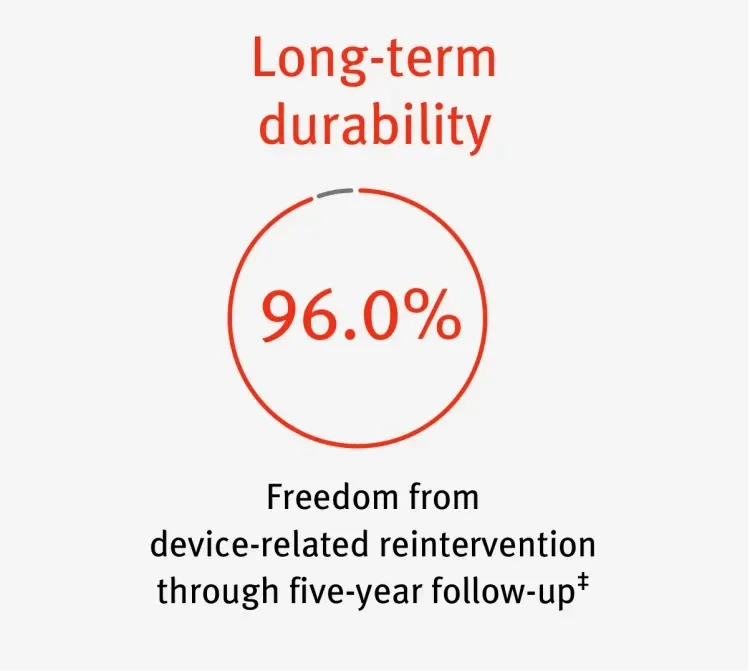
The GORE® EXCLUDER® Device family has consistently delivered low rates of reintervention for proven, durable value.*

Learn more about solutions that can help you improve patient outcomes
Value-based procurement: Shifting focus from costs to value creation and patient outcomes
Prioritizing purchase costs over process costs, purchasing price over delivery reliability, and low product costs over sustainability has dominated procurement for a long time. It's time for a paradigm shift that helps health care providers improve patient outcomes while operating economically. The key to this shift is value-based procurement.
 An example of how our medical devices can help create medical and economical value:
An example of how our medical devices can help create medical and economical value:
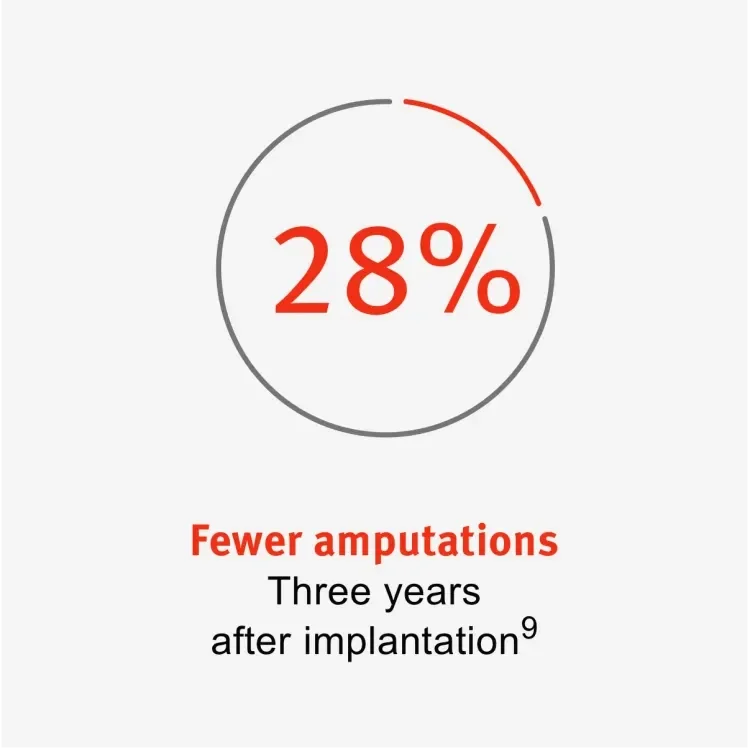
The GORE® PROPATEN® Vascular Graft is an artificial graft for peripheral arterial disease (PAD) patients. 33% of patients with critical limb ischemia need a major amputation within one year of diagnosis.6 There is a higher risk of death after amputation.7, § The long-term care costs after an amputation are significant; for example, 88,400€ per patient in Germany (2013).8
GORE® PROPATEN® Vascular Grafts could reduce amputations by 28% and could significantly decrease the need for reinterventions when compared to standard ePTFE grafts. This could lead to better patient outcomes, allowing health care providers to focus on other patients, potentially saving up to 37% in health care costs.9

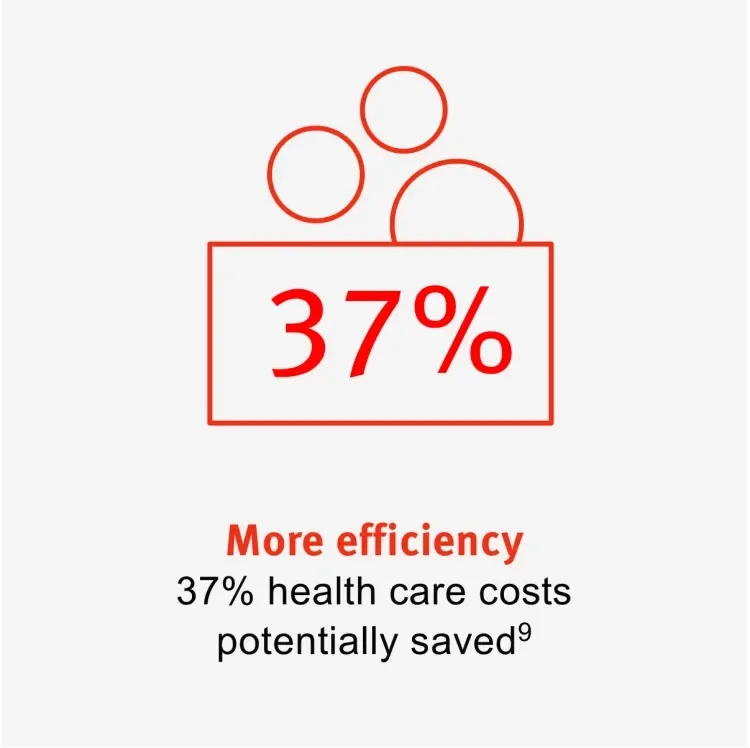
Learn more about solutions that can help you act more efficiently
A seamless supply chain experience from order to delivery
Our services deliver value even before you receive them: Gore's seamless product ordering and delivery process helps eliminate wasted time, money and worry by shortening the path from our warehouse to your O.R.
Streamlined delivery saves time and minimizes inventory



Electronic data interchange (EDI) saves time and reduces complexity

Reliable supply


Responsibility for better health and a better future
The environmental and social impact of our products and services are just as important as the value they offer. Our focus on sustainability is an expression of our promise – Together, improving life – and our long-established values, culture, principles and commitment to associates, customers and communities.
Our responsibility focuses on three areas:
- Environment - Minimizing our impact on the planet
- Social - Valuing people
- Engaging business - Practices for sustainable prosperity
High-quality vascular, cardiac, general surgery devices
Contact us to learn more about what we can do for you as a health care provider
* Based on U.S. clinical studies and post-approval registries.
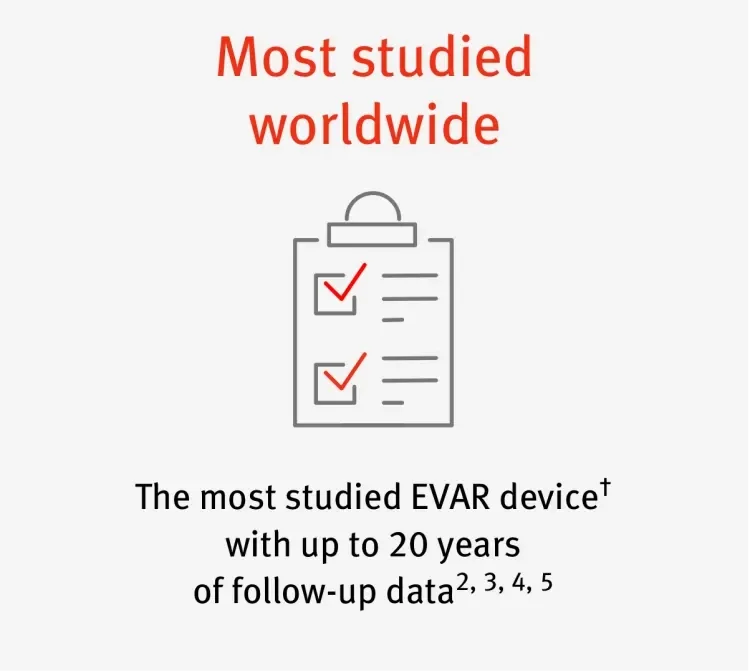
† Based on company-sponsored trials and registries shown on clinicaltrials.gov for currently available stent grafts.
‡ GREAT. n = 3,274. To calculate the overall event rates from procedure through end of study period, all subjects who could have had events, regardless of length of follow-up, were included. For outcome data, GREAT only collects site reported serious adverse events.
§ 99.2% of amputee patients had PAD
- Shaw SE, Preece R, Stenson KM, et al. Short stay EVAR is safe and cost effective. European Journal of Vascular & Endovascular Surgery 2019;57(3):368-373.
- Van Gool F, Houthoofd S, Mufty H, Bonne L, Fourneau I, Maleux G. Long-term outcome results after endovascular aortoiliac aneurysm repair with the bifurcated Excluder endoprosthesis. Journal of Vascular Surgery 2022;75(6):1882-1889.e2.
- Pratesi G, Piffaretti G, Verzini F, De Blasis G, Castelli P, Pratesi C. Ten-year outcome analysis of the Italian Excluder Registry with the Gore Excluder endograft for infrarenal abdominal aortic aneurysms. Journal of Vascular Surgery 2018;67(3):740-746.
- Poublon CG, Holewin S, van Sterkenburg SMM, Tielliu IFJ, ZeebregtsCJ, Reijnen MMP. Long-term outcome of the GORE EXCLUDER AAA Endoprosthesis for treatment of infrarenal aortic aneurysms. Journal of Vascular & Interventional Radiology 2017;28(5):637-644.e1.
- Atkins E, Milner R, Delaney CL; Global Registry for Endovascular Aortic Treatment (GREAT) Participants. Raised BMI is associated with fewer Type I endoleaks in patients treated with the Gore Excluder device: data from the Global Registry for Endovascular Aortic Treatment (GREAT). Journal of Cardiovascular Surgery 2023;64(5):513-520.
- Murphy TP. Medical outcomes studies in peripheral vascular disease. Journal of Vascular & Interventional Radiology 1998;9(6):879-889.
- Stern JR, Wong CK, Yerovinkina M, et al. A meta-analysis of long-term mortality and associated risk factors following longer extremity amputation. Annals of Vascular Surgery 2017;42:322-327.
- Hoffmann F, Claessen H, Morbach S, Waldeyer R, Glaeske G, Icks A. Impact of diabetes on costs before and after major lower extremity amputations in Germany. Journal of Diabetes & its Complications 2013;27(5):467-472.
- Iqbal K, Schumann E. Cost-consequence analysis of EPTFE vascular grafts with heparin end point covalent bond compared to standard EPTFE vascular grafts in below-knee surgical bypass for critical limb ischemia PAD patients in Germany. Presented at ISPOR Europe
(International Society for Pharmacoeconomics and Outcomes); November 6-9, 2022; Vienna, Austria. Value in Health 2022;25(12)Supplement:S114. EE306.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231268560-EN