Meeting the needs of complex peripheral artery disease: The devices and decisions needed for optimizing outcomes
Best practices for distal bypass using GORE® PROPATEN® Vascular Graft
The patient and case dynamics to consider when utilizing a surgical approach to PAD.
Richard Neville, MD, FACS
What are the dynamics that you take into consideration when deciding whether to treat surgically or endovascularly?
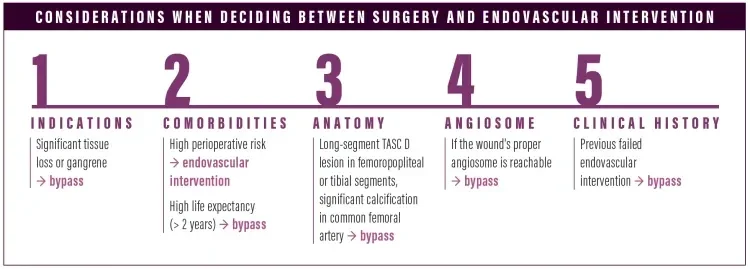
When we’re trying to decide whether bypass or an endovascular approach is appropriate, we take five particular things into consideration: (1) indications for the procedure: significant tissue loss or gangrenous changes would do better with bypass; (2) the patient’s medical comorbidities, including their perioperative risk and life expectancy: high perioperative risk would do better with an endovascular approach, versus someone with a significant life expectancy who would undergo bypass1; (3) the patient’s arterial anatomy: long-segment TASC-D lesions in the femoropopliteal or tibial segments or significant calcified common femoral artery disease would tend towards bypass2; (4) the wound's angiosome: all other things being equal, I might do a bypass to get to the proper angiosome; and (5) previous failed endovascular therapy.
Once you decide to treat surgically, what is the next key decision you make?
The next decision point in planning a bypass is inflow, outflow, and the conduit. For distal bypass, I try to use the patient’s own great saphenous vein if it’s available, ipsilateral or contralateral. If the patient does not have the great saphenous vein available, my next choice is to use the GORE® PROPATEN® Vascular Graft, and I use a vein patch at the distal anastomosis.
I’m no longer using arm vein or lesser saphenous vein, except in young patients with a long life expectancy, and I’m no longer doing composite PTFE and spliced segments of vein. The distal vein patch technique has proven superior to composite PTFE with spliced vein segments. I don’t use much cryopreserved vein—I reserve cryopreserved conduit for infected situations.
How do you determine the distal anastomosis technique?
When using the GORE PROPATEN Vascular Graft, I suture the proximal anastomosis directly to the artery, unless the patient has significant femoral artery disease, in which case a femoral endarterectomy may be needed. For a femoral endarterectomy, we put a patch on the endarterectomy and then put the GORE PROPATEN Vascular Graft into the patch. The distal anastomosis is usually to a small tibial artery, and I think you need the distal vein patch. We suture the patch to the artery with 7-0 MEDLINE PROLENE® Polypropylene Sutures, and then we suture the GORE PROPATEN Vascular Graft to the patch with 6-0 MEDLINE PROLENE® Polypropylene Sutures.
What is the clinical benefit of using the distal vein patch and GORE PROPATEN Vascular Graft?
Use of the GORE PROPATEN Vascular Graft with a distal vein patch combines the antiplatelet, antithrombotic, and antihyperplastic characteristics of heparin bonding with the advantages of the vein patch technique. These advantages include hemodynamic and compliance optimization as well as technical ease of suturing to small, calcified tibial arteries.3-5
Another benefit is if the GORE PROPATEN Vascular Grafts thromboses when you have used the distal vein patch technique, the failure occurs between the graft and the vein, not between the graft and the artery, making secondary patency much easier to establish. If you took the GORE PROPATEN Vascular Graft right to the artery and the graft fails, then the clot that develops extends into the artery. Using the GORE PROPATEN Vascular Graft and distal vein patch technique, if the artery doesn’t get thrombosed, I can expose the distal anastomosis under local anesthesia and reestablish flow and maintain secondary patency.
How might the treatment goals for your patients impact conduit selection and why?
The real goal is amputation-free survival. When using a graft to treat femoropopliteal disease, you can treat claudication and lifestyle, but for tibial bypasses, the goal is limb preservation and wound healing.
Are there other options you consider?
There are advanced endovascular techniques being developed that may impact this decision, but for right now I hold to my original indications.
DISTAL BYPASS BEST PRACTICES
What are the sources of vein patch that you use?
Despite long segments of vein that are atretic and suboptimal, there is usually a segment of saphenous vein that can be used—only 2 to 4 cm of vein is required. If not, then I’ve taken occluded superficial femoral artery and opened it longitudinally and performed an endarterectomy. You could also use superficial femoral vein.
I’ve rarely used lesser saphenous vein. We have not used arm vein. I do not use bovine pericardium for the patch; I’ve found that it leads to suboptimal results compared to a distal vein patch. There is also the AZIYO® CORMATRIX® ECM Vascular Repair Patch, which we have written about in other situations, but I would not use it in this setting.
When you make the distal anastomosis, what is your consideration for the venotomy site?
When we do the venotomy and construct the patch, we offset it to the proximal two-thirds of the patch. I want a third of the patch to be purely free of the PTFE.
What is the target artery diameter? Is there a limitation?
There is not a size limitation, but if we do a bypass to a very small tibial artery, we have come up with the Patchula technique, which adds a distal AV fistula to the anastomosis.6 If a target artery is < 1.5 to 2 mm, especially if arteriographic runoff is poor, I would consider a distal AV fistula.
What is the usual graft diameter?
I use a 6 mm graft. If I’m doing a fem-pop or a fem-fem, I use an 8 mm graft, but if I go below the knee, I use 6 mm.
Any tips on graft handling?
Surgeons probably know this, but I’ll catch nurses sometimes who will ask if we want the graft soaked—you don’t soak the graft. Also, keep the graft off the skin, we don’t want contamination.
What is your wound care protocol?
There is no one protocol, there are so many choices out there. We strongly advise that you involve podiatry, plastic surgery, and your local wound care centers and come up with a protocol that works for your facility.
What is your approach to patient follow-up regarding dual antiplatelet therapy and surveillance?
I do try to anticoagulate these patients. We have unpublished data that shows that if you anticoagulate them, there is a statistical trend toward improved patency. We usually use warfarin; we have also used the new oral agents, because of the issues associated with warfarin.
If patients cannot be anticoagulated for some reason, then we use dual antiplatelet therapy, aspirin 81, and Plavix. If the patient comes in on Plavix because of a coronary stent or another reason, then I don’t add anticoagulation and use dual antiplatelet therapy.
For every patient, even those I may discharge on dual antiplatelet therapy alone, in the hospital I put them on 48 to 72 hours of heparin during the initial, immediate postoperative period to establish flow through the graft.
For follow-up, we use the standard guidelines and image the grafts at 3, 6, and 12 months and then annually. We are also working on a new technology using microcensor technology and a remote monitoring system that gives a second-to-second evaluation of the graft and works remotely through Bluetooth applications.
What are your long-term expectations regarding quality of life and patency?
We published a 300 patient series in the European Journal of Endovascular and Vascular Surgery that found 50% patency at 4 years, but amputation-free survival was in the 70% to 80% range.4 I tell my patients that if I do a bypass, there’s a 50/50 chance it will be working over several years, but there’s a 75% to 80% percent chance that we’ll keep your leg and get the wounds to heal.
What happens if it fails? What would you do next? Would you have done anything differently?
If the graft fails we usually (unless the patient doesn't want to or is medically unstable) try to reestablish secondary patency through local anesthesia with a small incision at the distal anastomosis, and I think it’s much easier to do that with the GORE PROPATEN Vascular Graft and vein patch technique than other prosthetic grafts.
If native vein occludes, it’s hard to thrombectomize. The advantage to native vein is that the primary patency is better, but the disadvantage to native vein is that it is harder to reestablish secondary patency.
How does the GORE PROPATEN Vascular Graft design contribute to better outcomes for the patient?
The end-point covalent heparin bonding is the key. We think it’s much better than standard PTFE. The graft handles well, and works especially well with vein patch.
What is your approach to patient follow-up regarding dual antiplatelet therapy and surveillance?
I do try to anticoagulate these patients. We have unpublished data that shows that if you anticoagulate them, there is a statistical trend toward improved patency. We usually use warfarin; we have also used the new oral agents, because of the issues associated with warfarin.
If patients cannot be anticoagulated for some reason, then we use dual antiplatelet therapy, aspirin 81, and Plavix. If the patient comes in on Plavix because of a coronary stent or another reason, then I don’t add anticoagulation and use dual antiplatelet therapy.
For every patient, even those I may discharge on dual antiplatelet therapy alone, in the hospital I put them on 48 to 72 hours of heparin during the initial, immediate postoperative period to establish flow through the graft.
For follow-up, we use the standard guidelines and image the grafts at 3, 6, and 12 months and then annually. We are also working on a new technology using microcensor technology and a remote monitoring system that gives a second-to-second evaluation of the graft and works remotely through Bluetooth applications.
What are your long-term expectations regarding quality of life and patency?
We published a 300 patient series in the European Journal of Endovascular and Vascular Surgery that found 50% patency at 4 years, but amputation-free survival was in the 70% to 80% range.4 I tell my patients that if I do a bypass, there’s a 50/50 chance it will be working over several years, but there’s a 75% to 80% percent chance that we’ll keep your leg and get the wounds to heal.
What happens if it fails? What would you do next? Would you have done anything differently?
If the graft fails we usually (unless the patient doesn't want to or is medically unstable) try to reestablish secondary patency through local anesthesia with a small incision at the distal anastomosis, and I think it’s much easier to do that with the GORE PROPATEN Vascular Graft and vein patch technique than other prosthetic grafts.
If native vein occludes, it’s hard to thrombectomize. The advantage to native vein is that the primary patency is better, but the disadvantage to native vein is that it is harder to reestablish secondary patency.
How does the GORE PROPATEN Vascular Graft design contribute to better outcomes for the patient?
The end-point covalent heparin bonding is the key. We think it’s much better than standard PTFE. The graft handles well, and works especially well with vein patch.
- Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925-1934.
- Lawrence PF, Chandra A. When should open surgery be the initial option for critical limb ischaemia? Eur J Vasc Endovasc Surg. 2010;39:S32-S37.
- Neville RF, Tempesta B, Sidway AN. Tibial bypass for limb salvage using polytetrafluoroethylene and a distal vein patch. J Vasc Surg. 2001;33:266-272.
- Neville RF, Lidsky M, Capone A, et al. An expanded series of distal bypass using the distal vein patch technique to improve prosthetic graft performance in critical limb ischemia. Eur J Vasc Endovasc Surg. 2012;44:177-182.
- Neville RF, Elkins CJ, Alley MT, Wicker RB. Hemodynamic comparison of differing anastomotic geometries using magnetic resonance velocimetry. J Surg Research. 2010;169:311-318.
- Malas MB, Hicks CW, Jordan WD Jr, et al. Five-year outcomes of the PYTHAGORAS U.S. clinical trial of the Aorfix endograft for endovascular aneurysm repair in patients with highly angulated aortic necks. J Vasc Surg. 2009;50:83-88.
Products listed may not be available in all markets.
See all products by region:

Whether facilitating lower extremity revascularization or providing vascular access, the versatile GORE® PROPATEN® Vascular Graft is purposefully designed to improve outcomes and reduce reinterventions. With more than a decade of strong performance, this trusted vascular graft provides proven clinical and economic value.

A Decade of Performance
Visit propatenperformance.com to learn more.

Richard Neville, MD, FACS
Associate Director, Inova Heart and Vascular Institute Vice Chairman, Department of Surgery
Inova Fairfax Medical Campus
Falls Church, Virginia
Disclosures: Scientific advisory board for Gore & Associates.
