Meeting the needs of complex peripheral artery disease: The devices and decisions needed for optimizing outcomes
The when and how of PAD treatment: A roundtable discussion
A panel of PAD experts talk through the patient-specific factors and technical nuances that influence outcomes.
Dennis Gable, MD, FACS, RVT
Robert Beasley, MS, MD
Russell Samson, MD, FACS, RVT
Darren Schneider, MD, FACS
When to treat
Dr. Gable: Let’s first discuss the decision-making process for peripheral artery disease (PAD) as far as who you’re going to treat and when you treat them.
Dr. Beasley: Each patient is considered on a case-by-case basis. For example, I had a patient the other day who could not walk from the concourse in the airport to his car without stopping three times. He was fairly young and had an superficial femoral artery (SFA) occlusion—that is the kind of patient I want to treat. An older patient (75 or 80 years old) who has just a little bit of trouble because of SFA occlusions but has nice collateralization—that is the type who you want to start on a walking program and look at risk factor modification.
Dr. Schneider: We treat all patients with PAD, but we just don’t necessarily intervene on them all. We want to make sure we are treating the patients with asymptomatic PAD, not necessarily by offering them any interventions, but like you said—with risk factor modification and exercise programs that will hopefully make them better and prevent them from needing intervention.
Dr. Samson: In my belief, it is a shared decision-making. When you say, “What are we going to do for the patient?” it’s not what we’re going to do for the patient, but what the patient wants us to do for them. I think the most important thing is to understand exactly what the patient’s goal is and then offer them the different treatment options we have and inform them as best we can.
Dr. Beasley: The rapport with the patient is really important. You have to gauge how intensely the patient is suffering or how much they want an intervention.
Dr. Schneider: Dr. Samson’s point is important because with claudication, it’s subjective. There are some patients who can walk just two blocks, but they’re fine with that. Then there are some people who can walk two blocks, and it’s a massive disability that has a major impact on their job and daily life. You can’t know that unless you develop that relationship with your patient.
MEDICAL MANAGEMENT
Dr. Gable: Do you refer medical management out or keep it in your own practice? In either case, when do you follow up with the patient?
Dr. Beasley: I refer them to a cardiologist or internist.
Dr. Samson: If it’s a first-time patient with stable claudication, it would depend on whether I’m going to treat them medically or if I’m leaving it up to the internist. If I’m taking over the medical management, which I do in a small group of people, I see them every 3 months. If they’re under good care, I may see them in a year or 2 years.
Dr. Schneider: I do start patients on appropriate medications. If they’re not on antiplatelet and statin, I’ll start them myself. We communicate with their primary care doctor if they’re not already on those medications and should be. We counsel for smoking cessation, too. It is individualized, so it may be 3, 6, or 12 months before I’ll see them again. I reassure them that even if we’re not going to intervene, we’re going to follow them and make sure that they’re not getting worse.
REST PAIN
Dr. Gable: What do you do for patients who present with true rest pain (foot with dependent rubor but no tissue loss or ulcerations)?
Dr. Schneider: First, you must differentiate whether the patient is asymptomatic, whether they have some claudication, or whether they have critical limb ischemia (CLI). Rest pain is technically CLI, and that’s a critical decision point because for those patients with CLI, we’re definitely going to consider an intervention to prevent limb loss. For elderly debilitated patients with many comorbidities, endovascular intervention is preferable, but for multilevel disease or disease that may not lend itself to endovascular intervention, we also have to consider surgical intervention.
Dr. Beasley: It may also be a combination of some other disease modalities—venous, sciatica, neuropathy. It’s hard to gauge what is really pain. Figuring out if it’s truly arterial is a diagnostic conundrum. I would proceed to do an angiogram and then determine if there was something significant that would tip my hand one way or the other.
Dr. Samson: I think this terminology of chronic limb ischemia is a misnomer. People are talking about chronic limb threat rather than chronic limb ischemia. Many people with clinical typical rest pain, assuming it is vascular related, can go years before they get to ischemia that will cause them to lose their legs. You have to assess the risk of converting a stable situation into true limb ischemia. However, I think for someone who is complaining of rest pain, most of us are going to intervene if we have a good option for intervention.
Dr. Schneider: In addition to a good examination, it is important to do good physiologic studies as well—ankle-brachial indices (ABIs) (though for diabetic and renal failure patients, ABIs may be falsely elevated), toe pressures, looking at waveforms, and making sure that intervention is truly appropriate. We get physiologic studies on everyone in whom we’re going to intervene. You need to establish a baseline to support the diagnosis and then measure if you’ve had a treatment effect.
Dr. Samson: We should also involve the podiatrist in this decision tree, especially when you get into the chronic limb threat patient. Good podiatric care is critical.
TISSUE LOSS
Dr. Gable: How do you treat patients with tissue loss? Is there anything different you would use for diagnosis on your initial evaluation that you wouldn’t have done for someone with just rest pain?
Dr. Schneider: When you have a combination of tissue loss or a wound and documented arterial insufficiency, that’s where individualized treatment comes into play. By and large, they are going to get an intervention, whether it’s surgical or endovascular. As part of our workup, when we’re doing our arterial duplex examinations to assess the level of disease, we’ll usually do vein mapping so we know whether they have a good autologous saphenous vein conduit. If I think I’m likely to intervene, I usually go straight to angiography with the possibility of doing the endovascular intervention at that time.
Dr. Beasley: We almost never do CTAs or MRAs. We’ll make our decisions on the patient presentation in combination with a wound care specialist and physiologic studies. Depending on renal status, I usually shoot an angiogram first from a contralateral approach on the uninvolved side to get an idea of whether there is significant SFA or tibial disease. We’ll plan intervention 1 or 2 days later, likely going antegrade with a retrograde approach.
Dr. Samson: For proximal disease, I think CTA is essential. I image from the arch down because I am a believer in axillofemoral bypass grafts for people who have hostile aortas.
Dr. Schneider: There is value in CTA and MRA, especially when you are not sure about the anatomy or initial treatment approach based soley upon presentation and ultrasound studies. I prefer CTA because it helps me assess calcification when we’re looking at the aortoiliac system, common femoral arteries, and profunda. You do have to take renal function into account because so many of our CLI patients have impaired renal function.
How to treat | Surgical bypass
Dr. Gable: What comes into play when you are trying to choose between vein bypass and prosthetic bypass?
Dr. Schneider: It’s primarily the quality of the vein. If you have a good 4 mm saphenous vein, nothing beats that, and I still prefer vein for infrainguinal bypasses. If you have a borderline-quality vein and it’s an above-knee fem-pop, I’m probably going to choose a GORE® PROPATEN® Vascular Graft for that. There are a fair amount of data suggesting that the heparin-bonded GORE PROPATEN Vascular Graft has good patency for femoropopliteal bypass even below the knee. There are also a fair amount of data to suggest it’s better than plain polytetrafluoroethylene (PTFE), so I tend to use the GORE PROPATEN Vascular Graft if I’m doing a prosthetic lower extremity bypass.
Dr. Samson: Our data showed that the GORE PROPATEN Vascular Graft is better than uncoated ePTFE grafts, and that has held up across multiple studies. However, the fact that it’s better than standard ePTFE doesn’t mean that suddenly we use this when we wouldn’t have used ePTFE before.
As far as the above-the-knee popliteal, our prosthetic data show patients who are young do not do as well as patients who are older. If the patient is young, has excellent distal vessels, is not diabetic, and has a good vein, definitely use vein above the knee. If the vein is bad, then there is no decision here. I would not use arm vein. If they are diabetic and have severe distal disease, even if they’re young, I would use above-knee GORE PROPATEN Vascular Graft to save the saphenous vein for the future.
I would very rarely use saphenous vein from the other leg for bypass on an ipsilateral problem. If I had to use saphenous vein from the right leg to do an above-knee fem-pop on the left side, I wouldn’t use vein, I’d use the GORE PROPATEN Vascular Graft.
Dr. Schneider: Our approach is almost identical. I still prefer vein whenever possible, especially if there is a good ipsilateral saphenous vein. I will also take contralateral saphenous vein, unless the opposite limb needs a bypass, too. For an above-the-knee fem-pop, however, I generally would not take a contralateral saphenous vein because the GORE PROPATEN Vascular Graft data for the fem-pop are good.
Dr. Samson: For below-knee fem-pops, we would use saphenous vein preferentially if there’s a good saphenous vein, even if it means coming from the other side. If I don’t have a good vein, the GORE PROPATEN Vascular Graft had an approximately 58% patency rate at 5 years, which is good for a below-knee procedure.
INFECTION
Dr. Gable: If there is active infection, perhaps from a previous incision site, what is your choice of treatment?
Dr. Schneider: In a patient scenario like that, using a prosthetic there is going to be a potentially significantly elevated risk of graft infection. We are going to look for a vein, even arm vein, to try to get an adequate conduit to do an autologous bypass in a patient with infection.
Dr. Samson: A graft infection is a life-threatening condition. I would never put a prosthetic into a patient who is febrile or into a patient in whom I don’t believe I’ve controlled the infection. The white blood cell count would have to be as close to normal as possible, the patient should be afebrile, and I’ve done everything I can to control the local infection.
Dr. Gable: It’s been my experience that most of those patients, even though they’re presenting acutely, can usually tolerate 1 or 2 weeks of antibiotics and any other treatment needed first.
VEIN PATCH USE/SURGICAL TECHNIQUE
Dr. Samson: I do not use vein patches for fem-pop bypass. However, for tibial arteries, I have started to use the Neville patch. I think the patch works because it makes the distal anastomosis so easy. Putting a little piece of vein into the artery first makes that junction of the PTFE to the vessel so much easier technically.
Dr. Schneider: I completely agree. I don’t always use patches for below-knee popliteal bypasses, and it depends on the vessel size. I do use patches for the tibial arteries, especially since it can be difficult to sew an ePTFE graft to the smaller tibial arteries. The vein patch widens the target and probably results in less trauma to the tibial artery.
Dr. Gable: We’ll do that for small tibial vessels. For normal, good-size tibial arteries, I usually do not use a patch, but I’ll make my anastomosis a lot longer (a couple of centimeters) than normal.
Dr. Samson: It’s interesting how many referring physicians and vascular surgeons think of this operation as if it’s this straightforward, easy procedure. However, there is so much to consider. How long an anastomosis do you make? What suture do you use? How do you handle the artery? How do you cut the artery? Do you start from the common femoral? Do you start from the SFA? There are so many different variations how you can do a fem-pop.
Dr. Schneider: You can’t underestimate the technical aspects of these revascularizations. It has to be technically precise, and there are multiple steps and variables that go into conducting a proper operation with an optimal outcome. Bad technique leads to bad results, especially with tibial bypasses. In the small-caliber tibials, the toe of the anastomosis has to be perfect so that you don't restrict the outflow, which significantly increases the risk of graft failure.
Dr. Gable: Especially when you’re talking about a prosthetic tibial with the size mismatch, it’s paramount that every stitch is perfect.
COST CONSIDERATIONS
Dr. Schneider: There are cost-related downsides to both bypass and endovascular intervention. With bypass, you have the potential for wound morbidity, which can lengthen hospital stays and risk prosthetic graft infection. You also have patients who aren’t discharged to home but to rehab facilities for several weeks to recuperate, even after a simple bypass with two incisions, especially if they are frail and have comorbidities. Those costs must be factored as well and may offset some of the added device costs for endovascular therapy.
Dr. Beasley: Also, not all surgeons have the same technical skills. If a surgeon does an above-the-knee bypass and it failed, you add significant cost to the situation to go back and either do a redo bypass or to try to salvage.
Dr. Gable: It’s hard for me to ever argue against surgery because that’s what I do, but there is consideration for time lost from productivity and work, or for patients who are retired, time until back to normal daily life. It’s going to be a lot quicker with an interventional approach versus surgical approach. That’s not a reason, in and of itself, to make for a choice of treatment, but it is a consideration.
Dr. Schneider: People use plain old balloon angioplasty instead of using drug-coated balloons and use bare-metal stents because they are less expensive. This is a real conflict in office-based labs, where profitability can affect clinical decision making. It is influencing the delivery of care in the United States and whether patients are getting what we all believe may be the optimal treatment or a less expensive, and possibly less effective treatment.
Dr. Gable: Hospitals are trying to be viable with Centers for Medicare & Medicaid Services (CMS) reimbursement alone, so any product on the market needs to be a viable option under CMS reimbursement.
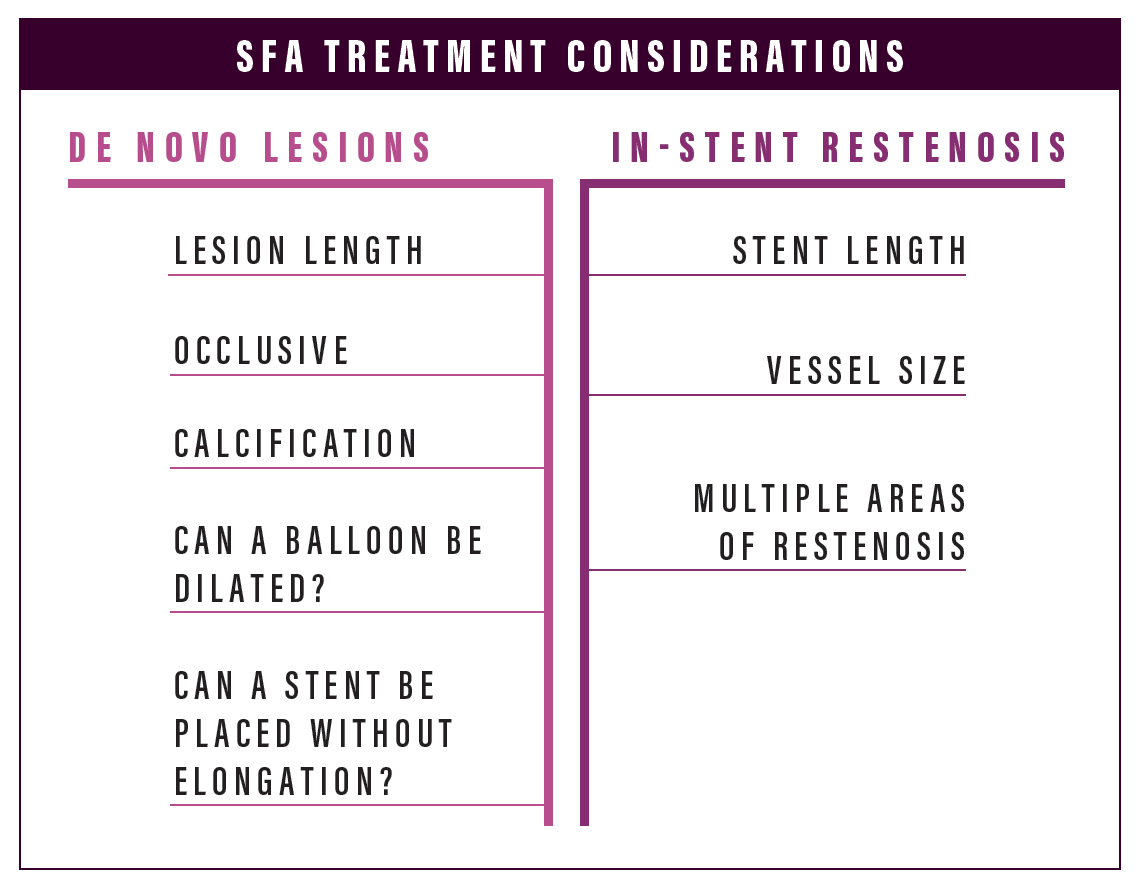
How to treat | De novo lesions
ILIAC ARTERIES
Dr. Gable: How do you approach treatment of iliac disease?
Dr. Samson: I usually use a bare-metal stent, but if I’m going to try to redo the bifurcation, I like to use covered stents because I know I’m going to want to come over from the other side at some stage, and I don’t want to have to worry whether I’m going through previously implanted stent struts.
Dr. Schneider: Certainly for me a covered stent is the first-line approach for long iliac occlusions, as well as for heavily calcified eccentric plaque, where I think there’s an elevated rupture risk. Covering that lesion up front is going to provide an additional layer of safety. Some data also show that patency of covered stents may be better than bare-metal stents for kissing stents at the bifurcation.
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft) is a game-changing device, because it is unique in marrying the properties of self-expanding and balloon-expandable covered stents in a single device. It can handle vessel tortuosity, has good radial force, and you can deploy it precisely. It also gives you the ability to flare the ends to different diameters, which can help in certain iliac anatomies.
Dr. Gable: For any type of significant calcification, especially in the bifurcation, I think a covered stent is the way to go.
SFA
Dr. Gable: What is everyone’s usage of different products for de novo disease in the SFA?
Dr. Schneider: If there is claudication and a long-segment SFA occlusion, even with some significant calcification, I’m going to do an angiogram and see if I can cross the lesion. If I can cross the lesion, then endovascular is still in the equation. If I cross the lesion but can’t adequately predilate the lesion, then that’s going to affect whether I’m going to use the GORE® VIABAHN® Endoprosthesis. I may opt for an alternative device that has more resistance against radial recoil in a heavily calcified lesion. If I can predilate adequately, then it’s still a good case for the GORE VIABAHN Endoprosthesis. If I can’t cross or predilate the lesion, then it’s not a good endovascular candidate, so I stop and we bring the patient in for a bypass. For every case, there is a plan A, plan B, and plan C, and you can make those decisions on the fly as long as they are all part of your algorithm.
Dr. Beasley: I say this as an interventionalist. We get to that point you just described, where there is four-quadrant calcification and even if you ballooned it, you know that you still won’t get a nice enough dilatation to put a bare-metal stent in without the stent elongating. Even if you put a 7 mm balloon in and try to open it up, you still have that recoil. A lot of interventionalists will put in a good GORE VIABAHN Endoprosthesis or bare-metal stent hoping that they will get some long-term patency. I think the best case scenario for that patient is to abort and refer the patient for bypass.
Dr. Schneider: If you do the procedure correctly and the patient has appropriate anatomy for the device, it is equivalent to a prosthetic fem-pop bypass.1 So for me, that is the first treatment option. Having appropriate anatomy for the procedure is important—you’ve got to be within the instructions for use with your sizing; you can’t be oversized and you need to have reasonable runoff.2 I rarely do above-knee fem-pop bypass unless a patient has already failed an intervention, and the lesion wasn't a good candidate for endovascular therapy in the first place.
Dr. Gable: When I talk to some interventionalists who use the GORE VIABAHN Endoprosthesis and end up saying they don’t like it, a lot of them are just not sizing appropriately, they’re not treating appropriately, they’re ballooning outside of the device. I think if you do the procedure right, place the device to a vessel that is non-diseased, and adhere strictly to the sizing criteria and techniques of deployment, you will get reproducible results. If you don’t do that, you will certainly see a failure of the device, but that is not really a failure of the device as much as it is a failure of the interventionalist.
Dr. Schneider: If you talk about the sweet spot for GORE VIABAHN Endoprosthesis use, it is cases where you have a complex clinical situation and challenging anatomy, especially a long occlusion, where you know most of the other endovascular alternatives are just not going to perform well. In those complex cases, there is clear justification for taking on the added cost of a covered stent.
Dr. Gable: There is a lot of push now for DCB use in long lesions, and there are some reports of patency rates of 80% to 90% at 1 year. What do you all think of that?
Dr. Beasley: I think we need long-term DCB data. The mantra these days is “leave nothing behind.” We don’t know yet, but I want to see DCB long-term data with 2 or 3 year follow-up.
Dr. Beasley: I use laser atherectomy a lot, and then I would use DCB and spot stent if necessary.
Dr. Gable: We rarely use laser in our group. There is some limited atherectomy use (rotational or orbital) either in an isolated popliteal lesion or a focal calcific lesion near endpoint. I personally never use atherectomy; I just don’t believe in it. The published data on it are typically < 12 months. Until you can show me longer-term data, I’m not going to buy into it.
Dr. Schneider: If you look at the data, there is no added benefit that’s been demonstrated of atherectomy over other modalities. Maybe there is a role for improving drug uptake and modifying a vessel that is heavily calcified so that it will become susceptible to treatment with a drug strategy. This still hasn’t been clearly demonstrated. The complication rate is also radically different between balloon alone versus balloon with atherectomy. There are cases of distal embolization and perforation.
Popliteal Segment
Dr. Gable: Does the GORE® TIGRIS® Vascular Stent play a role in any of your popliteal lesions?
Dr. Schneider: I don’t have a huge experience with the GORE® TIGRIS® Vascular Stent, but I do think that it is a good device for the popliteal. There are some data starting to come out on that; it is an incredibly flexible device, and you don’t have the same concerns with covering the entire genicular network. It’s easy to deliver and deploy accurately.
Dr. Gable: I’ve used the GORE TIGRIS Vascular Stent and I think it’s a great flexible stent. I only use it on a < 8 cm lesion if I was going to use it anyway, or to treat the popliteal artery. I think the popliteal artery is going to be our sweet spot for the GORE TIGRIS Vascular Stent.
TIBIAL VESSELS
Dr. Gable: If you have lesions in the tibial vessels, and you’re going to treat endovascularly because the patient is a poor operative candidate, what is your treatment method?
Dr. Samson: I use laser atherectomy and a very low-profile balloon. I’ve never stented in the tibials.
Dr. Beasley: I would probably use a dual approach, antegrade and retrograde, to get through the occlusion. I would then use microportal atherectomy and then a low-pressure balloon. I can’t say I never stent, but it would have to be in the proximal third, in an area that was a raging dissection.
Dr. Schneider: Primarily low-profile balloon angioplasty for tibial lesions. I do some bailout stenting with coronary drug-eluting balloon-expandable stents for spot areas, especially at vessel origins, but I do not line the entire tibial vessels with them. At this point, I don’t think that there is a significant role for covered stents in tibial arteries.
Dr. Gable: For tibial vessel disease, we will occasionally use low-profile laser atherectomy, and we will sometimes use coronary drug-eluting stents. Those are usually reserved for people who are not operative candidates. All those patients have either an angiogram or arterial duplex for follow-up. Surprisingly, the outcomes are good for the drug-eluting stents.
Dr. Schneider: I have started doing some tibial interventions in claudicants to try to improve runoff, but only if it’s a simple lesion that I think can be addressed with relatively low risk. If I have a focal, fairly tight tibioperoneal trunk stenosis, I’ll hit that with a balloon and I think the risk is pretty low. If we’re talking about crossing tibial occlusions and recanalizing occluded tibials, I’m not doing that routinely in claudicants.
I think that there is probably some role for atherectomy for a calcified eccentric lesion followed by angioplasty; and then if you don’t get the result or you have a dissection, then I would put in a short coronary drug-eluting stent.
How to treat | In-stent restenosis
Dr. Gable: What is your preferred method for treating in-stent restenosis?
Dr. Beasley: I think we are now able to prolong the inevitable, at least in years terms, with the GORE VIABAHN Endoprosthesis for in-stent restenosis. I have some patients who, years out after placing a GORE VIABAHN Endoprosthesis inside the stent, look pristine on ultrasound.
Dr. Gable: I go back and forth about debulking—currently, I do not. My preference is to reline with a GORE VIABAHN Endoprosthesis. If the original stent is 20 or 30 cm and the in-stent restenosis is only in a small section, then I may try to just use a DCB without debulking.
Dr. Samson: If I have a very long stent that is showing multiple areas of stenosis, I’m going to do a fem-pop bypass. If it is a short segment, I don’t see any reason why you wouldn’t try another endovascular approach.
Dr. Schneider: It depends on the length of the lesion and the length of the stent that’s in there. If it’s relatively short (10 to 15 cm), we have data showing that DCBs are effective in that lesion length, so we may use a DCB-first approach. If it’s an SFA full-metal jacket with diffuse in-stent restenosis, our preferred treatment is to reline completely with covered stents because we know that the data for covered stents in long lesions is good compared to a lot of the other technologies. I have had a number of patients who are out 4 or 5 years with patent GORE VIABAHN Endoprostheses after recurrent failures with a bare-metal stent.
Dr. Gable: Do you have any best practices for optimized outcomes when using a covered stent for in-stent restenosis?
Dr. Schneider: The caveat is that it has to be an adequate-size vessel to begin with. If you’re too oversized or dealing with small vessels, then it’s not a covered stent approach. We don’t debulk. We used to, and then we stopped, and we’ve had no difference in outcomes.
Dr. Gable: Do you think it makes a difference how far out at the end of the previous stent you put the GORE VIABAHN Endoprosthesis?
Dr. Schneider: You have to reline the entire stent. If you spot stent, the remaining uncovered nitinol stent will restenose again. We try to extend to get a normal artery landing zone, but that will be dictated by the patient’s anatomy.
- Reijnen MMPJ, van Walraven LA, Fritschy WM, et al. 1-year results of a multicenter randomized controlled trial comparing heparin-bondedendoluminal to femoropopliteal bypass. JACC Cardiovasc Interv. 2017;10:2320-2331.
- Weinstock BS. Optimal technique for use of the Gore® Viabahn® endoprosthesis. Endovasc Today. 2015;6(suppl 1):17-19.
Products listed may not be available in all markets.
See all products by region:

Moderator: Dennis Gable, MD, FACS, RVT
Chief of Vascular and Endovascular Surgery The Heart Hospital Baylor Plano
Plano, Texas
Disclosures: Consultant to and receives honoraria and research support from Gore & Associates and Medtronic.

Participant: Robert Beasley, MS, MD
Director of Vascular/Interventional Radiology and Vein Treatment Center
Mount Sinai Medical Center
Miami Beach, Florida
Disclosures: Speaker/trainer for Abbott Vascular, BD Interventional (also on medical advisory board), Boston Scientific Corporation (also on medical advisory board), Cordis/Cardinal Health, Cook Medical, Cardiovascular Systems, Inc., Endologix, Gore & Associates, Lake Region Medical, Medtronics, Philips/Spectranetics, Penumbra, Terumo/Bolton

Participant: Russell Samson, MD, FACS, RVT
Clinical Professor of Surgery
Florida State University Medical School Senior Surgeon
Sarasota Vascular Specialists
Sarasota, Florida
Disclosures: Received compensation from Gore & Associates for roundtable participation.

Participant: Darren Schneider, MD
Associate Professor of Surgery
Weill Cornell Medical College
Chief, Vascular and Endovascular Surgery Weill Cornell Medicine
New York-Presbyterian Hospital
New York, New York
Disclosures: Consultant for Gore & Associates and Medtronic; receives research support from Gore & Associates, Boston Scientific Corporation, and Cook Medical; receives honoraria from Gore & Associates and Abbott Vascular.


