Meeting the needs of complex peripheral artery disease: The devices and decisions needed for optimizing outcomes
Case report: GORE® TIGRIS® Vascular Stent implantation in segmental occlusion of the popliteal artery
Vascular-mimetic properties of this hybrid stent design contribute to high performance in a challenging vascular zone.
Jacob Sharafuddin
Brian Miller, MD
Mel Sharafuddin, MD
A 70-year-old man presented to our office with a history of progressive intermittent claudication and bilateral common iliac artery stenting years earlier. He had failed prior conservative management including cilostazol and risk factor management, but had been unable to comply with a walking program due to debilitating pain. He underwent angiography with unsuccessful attempted endovascular intervention for segmental occlusion of the right popliteal artery at an outside hospital. He described his current symptoms as debilitating, with exertional right calf pain occurring at half a block. He denied having rest pain or tissue loss. His vascular risk factors included diabetes, smoking, hypertension, and hyperlipidemia. His past medical history was also remarkable for stage 3 chronic kidney disease and atrial fibrillation. His examination was remarkable for diminished distal pulses. Resting right ankle-brachial index (ABI) was 0.59 with blunted transmetatarsal pulse volume recording (PVR).
Management options discussed with the patient included continued best medical management and exercise therapy, bypass surgery, and endovascular intervention. The patient elected to proceed with an endovascular approach.
COURSE OF TREATMENT
Given the patient's history of chronic kidney disease, the bulk of the angiographic runs were performed using CO2 with judicious use of diluted iodinated contrast medium. A contralateral right transfemoral approach was used for access. The presence of the previously implanted ostial iliac stents posed some difficulties that were overcome with the use of a tapered coaxial system and a flexible sheath, which enabled sufficient support for planned traversal of the known chronic total occlusion and subsequent delivery of the interventional devices.
Angiography demonstrated a 4 cm occlusion of the distal above-knee popliteal artery with an adjacent long segment of moderate calcific atherosclerosis in the proximal popliteal artery (Figure 1). The popliteal occlusion was traversed using a COOK® CXI® Support Catheter and a coaxial TERUMO® GLIDEWIRE® Stiff Shaft Guidewire. Intraluminal position within the reconstituted segment was confirmed, and an ABBOTT TAD II Tapered Guide Wire System was used for the remainder of the intervention. The occlusion and upstream stenotic segment were predilated using a 6 mm COOK® ADVANCE® ENFORCER 35 Focal-Force PTA Balloon Catheter. Angiography demonstrated restored patency to the occluded segment, but multiple intimal defects persisted across the treated segment. We proceeded with stenting using a 6 x 100 mm GORE® TIGRIS® Vascular Stent distally, overlapped proximally with another 7 x 100 mm GORE TIGRIS Vascular Stent. The stented segment was postdilated up to 6 mm.
RESULTS
Completion angiogram showed complete resolution of the occlusion with no residual stenosis across the stented length. Angiography was also performed at a 90° knee flexion position, confirming maintained patency and excellent adaptation of the stented segment to the adjacent infolding of the geniculate popliteal artery without any kinking (Figure 2). Assessment of the runoff confirmed wide patency of the infrageniculate popliteal artery and preserved three-vessel runoff to the foot. At completion of the procedure, the patient had strongly palpable pedal pulses. Postprocedural ABI and PVR were normal. At clinical follow-up 1 month after the procedure, the patient reported complete resolution of his previous symptoms with normal ABI and PVR values (Figure 3). A widely patent stent was confirmed on duplex sonography.
DISCUSSION
The femoropopliteal artery is a highly dynamic vascular segment subjected to repetitive multidimensional stresses, with multiple folding points and regions subjected to dynamic extrinsic compression.1 It also has a high prevalence of heavy calcification and total occlusions.
Our case illustrates the advantages of the GORE TIGRIS Vascular Stent for managing challenges encountered in the endovascular management of femoropopliteal artery occlusive disease. When compared to conventional nitinol stent designs, the dual-component design of the GORE TIGRIS Vascular Stent markedly enhances its ability to dynamically adapt to the vascular anatomy, mimicking the physiologic behavior of the native target artery (Figure 4).2 In addition, when compared to other vascular mimetic-type stents, it has the distinct advantage of extremely accurate deployment and long-axis adaptability with the lack of foreshortening or elongation. Other key advantages of this implant include high resistance to fracture and heparin-bonding to the interconnecting fluoropolymer mesh, providing local thromboresistance.3 These features represent the basis for the value of GORE TIGRIS Vascular Stent in the treatment of vascular segments associated with high mechanical stresses, particularly the popliteal artery.
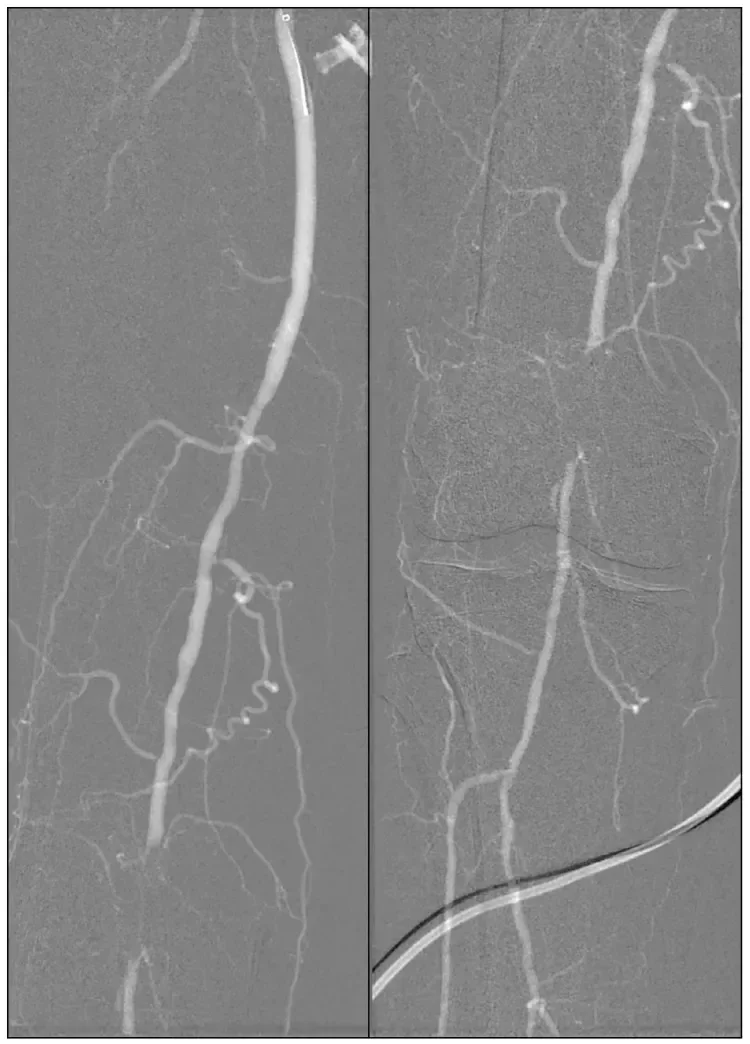
Figure 1. CO2 angiogram of the right lower extremity showing diffuse moderate disease of the above-knee popliteal artery with segmental occlusion at the level of the patella.
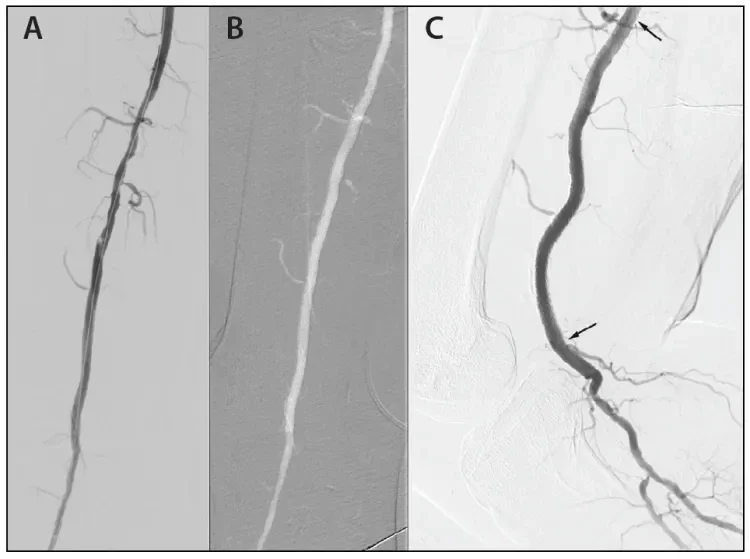
Figure 2. Residual irregularity in the popliteal artery after scoring balloon dilatation and deployment of the distal GORE® TIGRIS® Vascular Stent (A). Final angiogram after deployment of the proximal GORE® TIGRIS® Vascular Stent and postdilatation to show complete normalization of the popliteal artery lumen (B). Lateral angiogram (C) after knee flexion showing maintained patency and excellent conformation to the resulting infolding of the popliteal artery allowing gentle looping instead of kinking, which can be seen with conventional nitinol stents in Figure 4. Arrows indicate where the stent starts and ends.
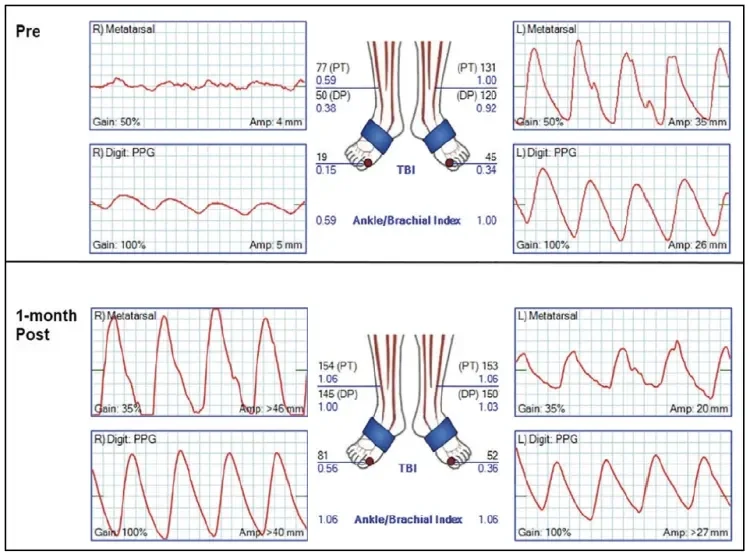
Figure 3. Noninvasive assessment 1 month after right popliteal artery stenting shows normal ABI and PVR values.
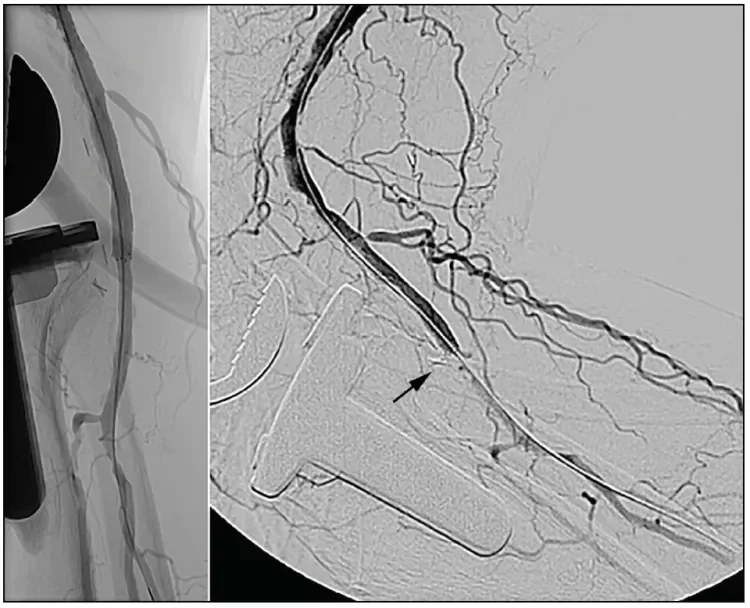
Figure 4. Inability of a conventional nitinol stent (BARD® LIFESTENT® Vascular Stent System) to accommodate the infolding of the popliteal artery, resulting in kinking on knee flexion.
Contraindications to the implantation of the GORE TIGRIS Vascular Stent, as with other nitinol stents, include failure to adequately predilate the stenotic lesion prior to stent deployment, very small target vessel diameter, poor distal runoff, or inability to stent a lesion without caging a vital branch.
The importance of overcoming a resistant stenosis prior to deployment of the GORE TIGRIS Vascular Stent cannot be overstated, especially in the setting of heavy calcification or the presence of fibrotic recoil. Our practice has been to routinely predilate lesions in the popliteal artery segment using a scoring or cutting balloon to ensure optimal deployment of the stent. This approach is very effective even in the presence of heavy calcification and obviates the need for lesion prepping using atherectomy devices.
- Maleckis K, Deegan P, Poulson W, et al. Comparison of femoropopliteal artery stents under axial and radial compression, axial tension, bending, and torsion deformations. J Mech Behav Biomed Mater. 2017;75:160-168.
- Parthipun A, Diamantopoulos A, Kitrou P, et al. Use of a new hybrid heparin-bonded nitinol ring stent in the popliteal artery: procedural and mid-term clinical and anatomical outcomes. Cardiovasc Intervent Radiol. 2015;38:846-854.
- Agarwal S, Pitcavage JM, Sud K, Thakkar B. Burden of readmissions among patients with critical limb ischemia. J Am Coll Cardiol. 2017;69:1897-1908.
Products listed may not be available in all markets.
See all products by region:
Jacob Sharafuddin
Iowa City Community School District
Iowa City, Iowa
Disclosures: None.
Brian Miller, MD
Departments of Surgery
The University of Iowa Roy and Lucille Carver College of Medicine
Iowa City, Iowa
Disclosures: None.
Mel Sharafuddin, MD
Departments of Surgery
The University of Iowa Roy and Lucille Carver College of Medicine
City
Iowa City, Iowa
Disclosures: Receives clinical trial grant support from Bolton Medical, Gore & Associates, Medtronic, Cook Medical, Terumo Interventional Systems, and Bard Peripheral Vascular.

