Advancing endovascular options: New endovascular devices and insights to extend aortic treatment to more patients
ADVANCING ENDOVASCULAR OPTIONS
Sponsored by Gore & Associates
Early experience using the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System: Why it is my preferred TEVAR device
A new TEVAR delivery system allows staged, predictable endograft deployment and in situ angulation control, while avoiding windsocking and bird-beaking.
Giovanni B. Torsello, MD
Martin Austermann, MD
Giovanni F. Torsello, MD, BA
The introduction of thoracic endovascular aortic repair (TEVAR) has completely changed clinicians’ attitudes and approaches in the treatment of thoracic aortic pathologies.1 Since the advent of TEVAR, we have witnessed endovascular repair being applied to treat increasingly complicated disease states in challenging aortic anatomies. Thoracic endografting provides a therapeutic option to an increasing number of patients who are not good candidates for open repair or who prefer a minimally invasive approach.2
Over the years, much progress has been made to improve TEVAR in terms of the design of both the stent graft and the delivery system. However, despite recent advancements, TEVAR is not free from complications, and challenges with bird-beaking and windsocking continue to be a problem, especially in the aortic arch. Failure to conform to arch anatomy may increase the risk of type I endoleak, which, if left untreated, can result in aortic rupture and possibly death. Windsocking due to high blood flow velocity and pressure in the aortic arch can lead to difficulties with precise deployment. This is especially true with deployment mechanisms where the proximal end opens while the distal end remains constrained. Predictability of deployment is also important at the level of the distal landing zone to avoid covering of the visceral arteries.
To overcome these challenges, the new GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System* introduces novel features that help to enhance deployment accuracy and stent graft apposition and to fully take advantage of the stent graft’s conformability.
TECHNICAL CONSIDERATIONS
The GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System combines the familiar Conformable GORE® TAG® Device with a novel nested handle delivery system. The stent graft remains the same as the Conformable GORE TAG Device and therefore has the same conformable stent graft design with sutureless graft attachment, oversizing windows that enable optimized radial fit, and indications and anatomic requirements. The principle of operation is also maintained, with a fiber deployment mechanism that is actuated to release the self-expanding stent graft from a constraining sleeve.
The delivery system, however, is different and now features a nested handle that separates stent graft expansion into two deployment steps, each requiring actuation of a separate handle component (Figure 1). Deployment consists of four required steps and two optional angulation steps. Removal of the primary deployment handle deploys the stent graft to its intermediate diameter, initiating device opening from the leading to the trailing ends (Figure 2). At this intermediate stage, the angulation control dial is now accessible and the user has the first opportunity for optional angulation of the proximal end of the stent graft by rotating this dial. Removal of the secondary deployment handle deploys the stent graft to its full diameter, initiating deployment from the trailing to leading ends. The angulation control dial remains accessible even after full expansion, and the user has a second opportunity for optional angulation at full diameter. Throughout the deployment, the stent graft is secured onto the catheter via a lock wire. This ensures control of device positioning throughout the procedure until removal of the lock wire handle. Finally, the angulation assembly handle is removed to withdraw the angulation fiber mechanism.
The GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System has two important features to improve deployment predictability through accuracy and control: staged deployment and in situ angulation control of the proximal end of the stent graft. With staged deployment, the nested handle delivery system design enables the stent graft to deploy in two distinct steps (Figure 3). Removal and actuation of the first primary deployment handle opens the device along its entire length to its intermediate diameter, which is approximately 50% of its nominal diameter. Importantly, this deployment to intermediate diameter is designed to allow continuous blood flow both through the lumen of the stent graft and around the device. This feature ensures hemodynamic stability throughout the procedure, even in the aortic arch with its characteristic high blood flow pressure and velocity. A unique advantage of this design is that blood pressure does not have to be aggressively reduced because the blood flow into the distal aorta is not compromised. The lack of windsocking forces that tend to push the device distally during device opening helps to ensure precise positioning of the stent graft in the aorta. Because there is little or no wall apposition at this intermediate diameter, it is possible to make refinements in the stent graft positioning. If required, it is also possible to modify the C-arm gantry angle to remove device parallax and maximize every millimeter of the landing zone. Removal and actuation of the secondary deployment handle opens the device to its full diameter. A lock wire secures the stent graft to the catheter, ensuring that the user maintains control of the device and its positioning throughout the procedure until the lock wire is removed as one of the final deployment steps.
Angulation of the proximal end of the stent graft is an optional feature that allows for more orthogonal placement of the stent graft. The benefit of angulation becomes evident in angulated aortic arch anatomies, where the wall apposition along the inner aortic curvature can sometimes lead to the formation of a bird beak. The GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System allows the user to adjust the angulation and orthogonal placement of the proximal end of the stent graft in situ, depending on the individual anatomic needs of the patient. Angulation control is possible during the two phases of deployment, at intermediate diameter and after the stent graft has expanded to its full diameter (Figure 4). Because this is an optional feature, the clinician can decide during deployment whether and to what degree angulation is beneficial in that specific anatomy. This ability to optimize proximal device apposition may help to avoid complications such as type Ia endoleak.
CASE REPORT
A 79-year-old man with a documented medical history of a type B aortic dissection presented with thoracic pain (Figure 5). A CT scan revealed an aneurysm of the false lumen and collapse of the true lumen (Figure 6). Endovascular exclusion of the aneurysm was deemed to be appropriate to prevent aneurysm rupture. TEVAR planning was based on a thoracoabdominal thin-slice CTA. The preoperative maximum outer-outer thoracic diameter was 57 mm. Proximal neck length and diameter were measured using the vessel centerline-of-flow technique.
The procedure was performed in a hybrid endovascular suite using fusion imaging under general anesthesia. The right femoral artery was accessed percutaneously using the preclose technique, which has been described elsewhere.4 During the procedure, intravenous heparin was administered to reach an activated clotting time corridor of 250 to 300 seconds. A 22-F GORE® DrySeal Sheath was placed. Under fluoroscopic guidance, two 34-mm-diameter GORE TAG Conformable Thoracic Stent Grafts were implanted. A permissive hypotension of < 100 mm Hg systolic blood pressure sufficed. The first 200-mm-long GORE TAG Conformable Device was placed with the proximal end just below the origin of the left subclavian artery (Figure 7). At intermediate diameter, it was observed that positioning of the device could be optimized by modifying the C-arm angle to remove device parallax.
The device was then deployed to its full diameter, and angulation of the endograft was performed. The device conformed to the aortic arch, with no bird beak on the inner curvature. A second device was placed for distal extension with the same diameter and a length of 10 cm, aiming for an overlap of 5 cm. After deployment of the stent graft, completion angiography was performed to ensure adequate placement of the graft and to rule out endoleaks (Figure 8). Postoperatively, mean arterial blood pressure was kept between 80 and 100 mm Hg in order to reduce the risk of ischemic spinal cord injury. Cerebrospinal fluid drainage was not performed, as we abandoned it as a prophylactic measure several years ago.5

Figure 1. The new GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System and associated accessory devices. The stent graft design remains the same as the previous-generation Conformable GORE® TAG® Device.
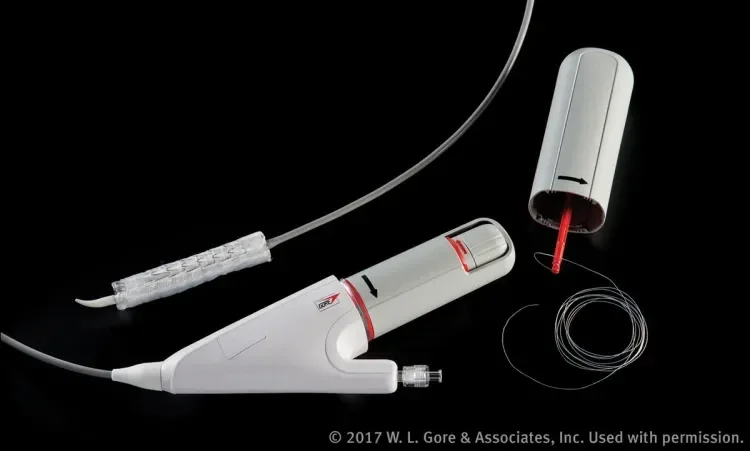
Figure 2. The nested handle of the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System enables stepwise deployment and optional angulation of the device. This figure shows the device deployed to its intermediate diameter after removal of the primary deployment handle.
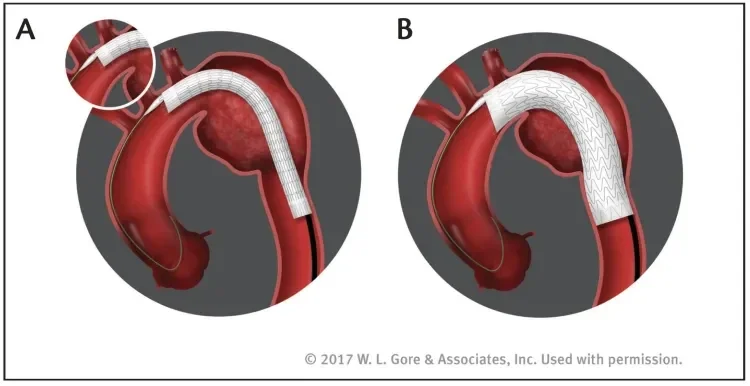
Figure 3. Staged deployment allows the device to first be opened to an intermediate diameter along its entire length while ensuring continuous blood flow (A). A separate deployment step expands the device to its full diameter (B).
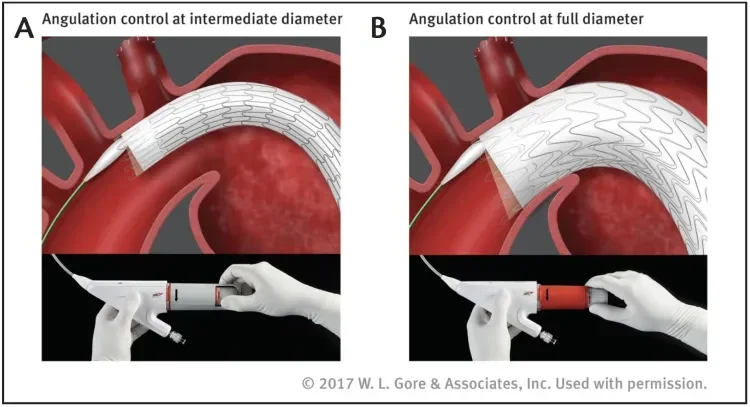
Figure 4. The optional angulation feature can be used at both intermediate (A) and full (B) diameter stages. By nesting the stent rows along the inner aortic curve, angulation helps to achieve orthogonal device placement in tight arches.
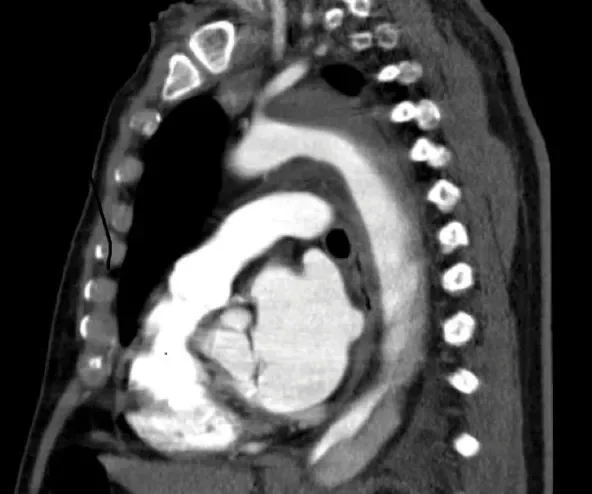
Figure 5. A CT scan showing a type B aortic dissection treated by best medical treatment.
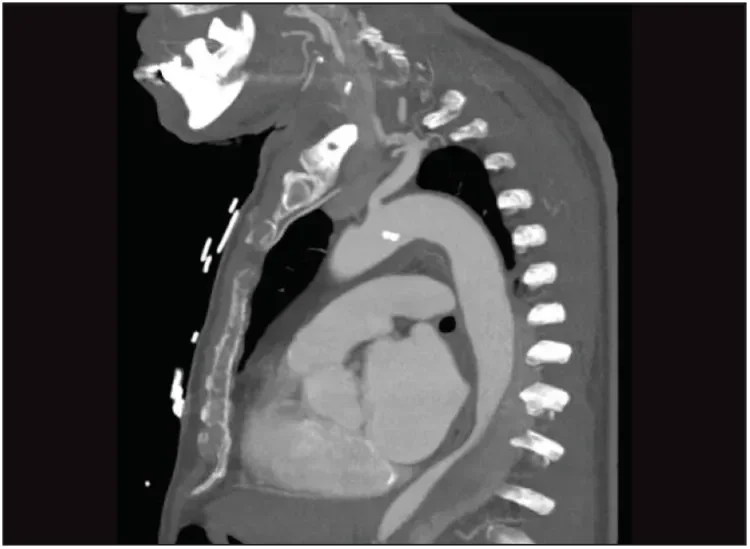
Figure 6. One year later, the patient presented with thoracic pain. The CT scan shows remodeling of the proximal descending aorta with an aneurysm of the false lumen and collapse of the true lumen.
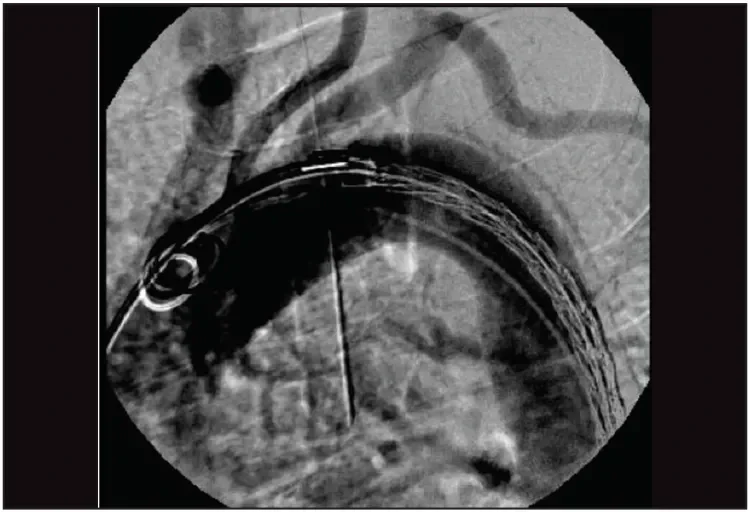
Figure 7. The first 200-mm-long GORE® TAG® Conformable device was placed with the proximal end just below the origin of the left subclavian artery and deployed to its intermediate diameter. The position of the device could be optimized by removing device parallax and adjusting the device position.
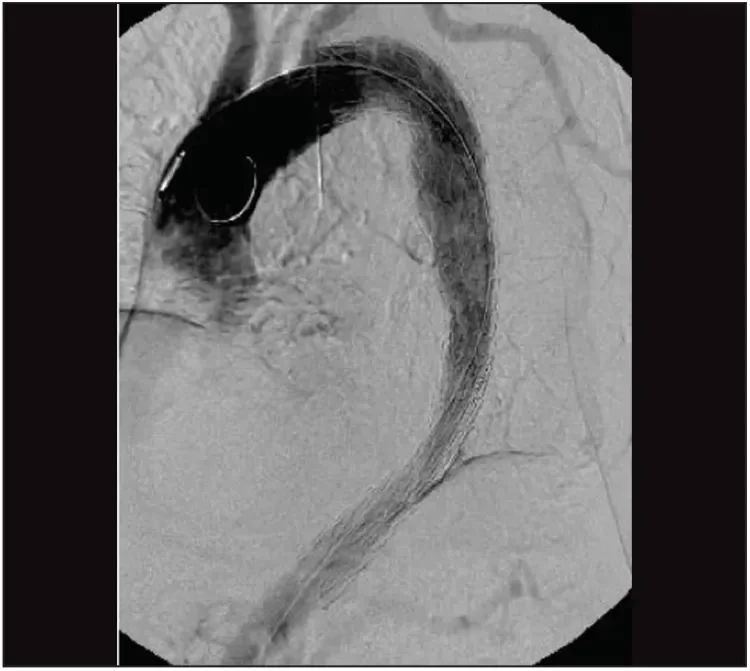
Figure 8. After deployment of the device to its full diameter, the completion angiogram shows adequate placement of the graft in the arch without any endoleak.
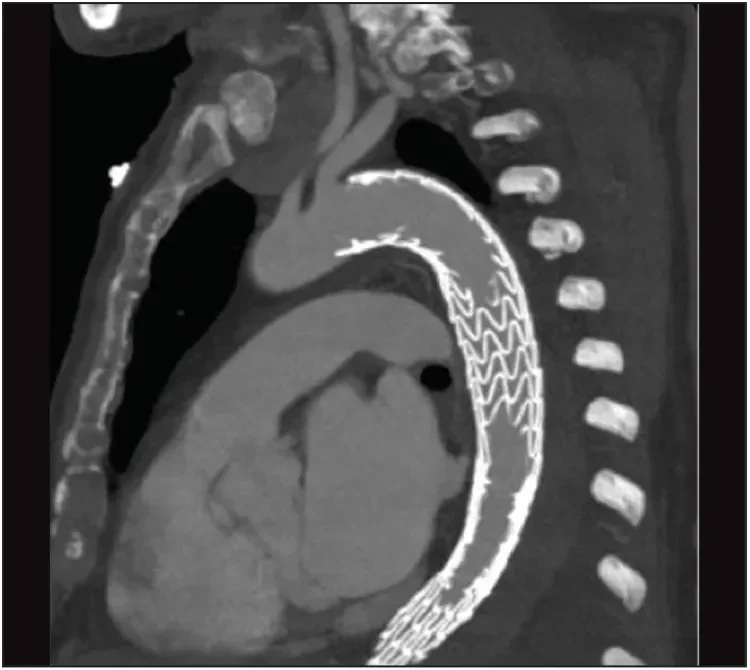
Figure 9. The postoperative CT scan shows exclusion of the aneurysm with no endoleak and good perfusion of the left subclavian artery.
A postoperative CT scan revealed exclusion of the aneurysm with no endoleak and good perfusion of the left subclavian artery (Figure 9) and visceral arteries. The patient had no neurologic complications and was discharged from the hospital a few days later. At 3 months, the patient is doing well. A control CT scan is planned 6 months after the procedure.
CONCLUSION
The Conformable GORE TAG Endoprosthesis was already widely recognized for its conformable stent graft design and its proven history across broad indications. With the new features of staged deployment and angulation control, the GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System represents a new development in TEVAR device design that enables in situ adjustments in stent graft positioning and apposition and sets a new standard for the conformability and control that can be expected of a TEVAR device. A prospective controlled registry, the SURPASS registry, will include the experience of up to 20 centers across Europe and will show the performance of the new system in the real world.
- Conrad MF, Cambria RP. Contemporary management of descending thoracic and thoracoabdominal aortic aneurysms: endovascular versus open. Circulation. 2008;117:841-852.
- Desai ND, Burtch K, Moser W, et al. Long-term comparison of thoracic endovascular aortic repair (TEVAR) to open surgery for the treatment of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2012;144:604-609; discussion 609-611.
- Panuccio G, Torsello GF, Pfister M, et al. Computer-aided endovascular aortic repair using fully automated two- and three-dimensional fusion imaging. J Vasc Surg. 2016;64:1587-1594.
- Eisenack M, Umscheid T, Tessarek J, et al. Percutaneous endovascular aortic aneurysm repair: a prospective evaluation of safety, efficiency, and risk factors. J Endovasc Ther. 2009;16:708-713.
- Bisdas T, Panuccio G, Sugimoto M, Torsello G, Austermann M. Risk factors for spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg 2015;61(6):1408-16
Products listed may not be available in all markets.
See all products by region:
Giovanni B. Torsello, MD
Chief of Vascular Surgery Department
St. Franziskus Hospital
Professor of Vascular Surgery
University of Münster
Münster, Germany
Principal Investigator of the SURPASS Registry
Disclosures: None.
Martin Austermann, MD
Vascular Surgery Department
St. Franziskus Hospital
Assistant Professor of Vascular Surgery
University of Münster
Münster, Germany
Disclosures: None.
Giovanni F. Torsello, MD, BA
Radiology Department
Charité Universitätsmedizin Berlin
Berlin, Germany
Disclosures: None.
