
CBAS® Heparin Surface.
Proven heparin bonding technology for lasting thromboresistance
The unique CBAS® Heparin Surface — a long-lasting heparin lining designed to resist thrombus formation.
See the technology below.
Heparin must be present, available and active to provide thromboresistance.
Without CBAS® Heparin Surface
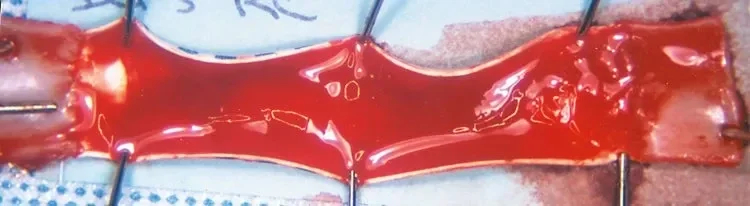
The 3 mm diameter control expanded polytetrafluoroethylene (ePTFE) graft is covered with thrombus in an acute two-hour in vivo canine carotid artery interposition model.
With CBAS® Heparin Surface

CBAS® Heparin Surface of a 3 mm diameter GORE® PROPATEN® Vascular Graft remains free of thrombus.
How it works
The anticoagulant function of heparin is dependent on the bioavailability of an active site within the molecule.
Proprietary covalent end-point bonding
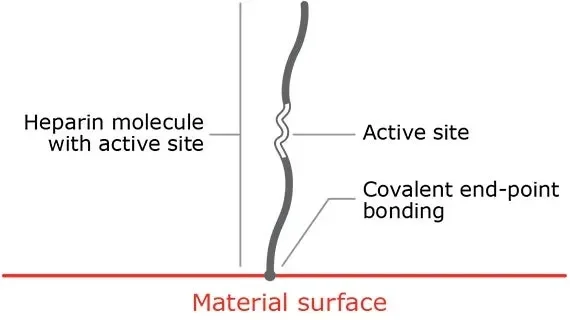
Covalent end-point bonding allows the heparin to extend into the bloodstream, keeping the active site bioavailable.
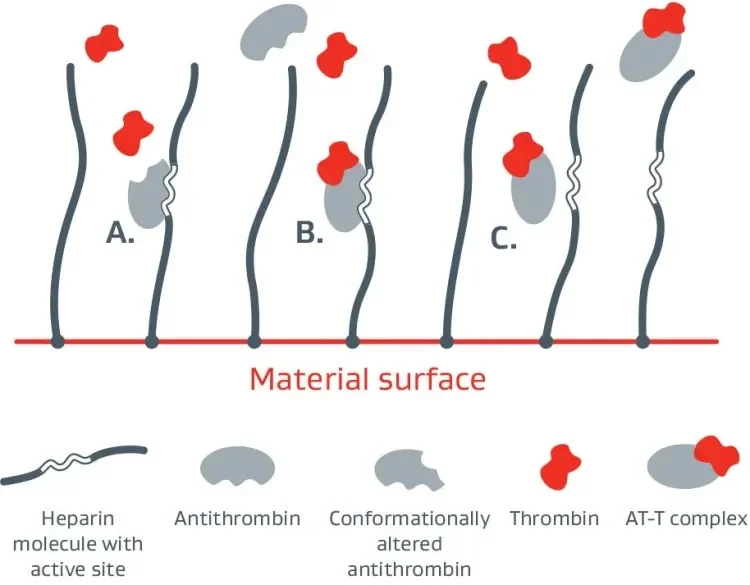
The CBAS® Heparin Surface lines the entire graft and keeps heparin anchored to the graft surface, to help prevent clotting.
A. Bioactive site of the heparin molecule enables antithrombin to bind thrombin.
B. When antithrombin binds to thrombin, a neutral thrombin antithrombin (TAT) complex is formed.
C. Neutral TAT complex detaches from the heparin molecule. Active site becomes available to again bind antithrombin.
Featured Gore products with CBAS® Heparin Surface
GORE® ACUSEAL Vascular Graft
GORE® PROPATEN® Vascular Graft
GORE® PROPATEN® Vascular Graft configured for Pediatric Shunt
GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
GORE® TAG® Thoracic Branch Endoprosthesis (Side branch component)
*As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
- Gore S, Andersson J, Biran R, Underwood C, Riesenfeld J. Heparin surfaces: impact of immobilization chemistry on hemocompatibility and protein adsorption. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2014;102(8):1817-1824.
- Begovac PC, Thomson RC, Fisher JL, Hughson A, Gällhagen A. Improvements in GORE-TEX® Vascular Graft performance by Carmeda® BioActive Surface heparin immobilization. European Journal of Vascular & Endovascular Surgery 2003;25(5):432-437.
- Freeman J, Chen A, Weinberg RJ, Okada T, Chen C, Lin PH. Sustained thromboresistant bioactivity with reduced intimal hyperplasia of heparin-bonded PTFE Propaten Graft in a chronic canine femoral artery bypass model. Annals of Vascular Surgery 2018;49:295-303. http://www.sciencedirect.com/science/article/pii/S0890509617310981
- Biran R, Pond D. Heparin coatings for improving blood compatibility of medical devices. Advanced Drug Delivery Reviews 2017;112:12-23. https://www.sciencedirect.com/science/article/pii/S0169409X16303210
- CARMEDA® BioActive Surface (also known as CBAS® Heparin Surface) Reference List. W. L. Gore & Associates, Inc. website. Updated April 2020. Accessed April 27, 2021. https://www.carmeda.se/selected-reading

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231237294-EN
