GORE® EXCLUDER® Iliac Branch Endoprosthesis
The GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE) is the only FDA approved, off-the-shelf iliac branch solution and is designed to preserve blood flow to external and internal iliac arteries.
See the results: IDE Study Outcomes. Five-year follow-up*
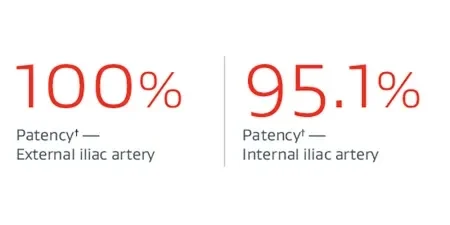
* U.S. IDE Clinical Trial. Sixty-three subjects with device implanted in initial cohort. Thirty-six patients have completed five-year follow-up.
† Core Lab reported assessment for patency, endoleak, migration and CIAA enlargement (>5mm). Denominator is number of subjects evaluated for primary effectiveness endpoint result with an evaluable result.
‡ On the side treated with the IBE.
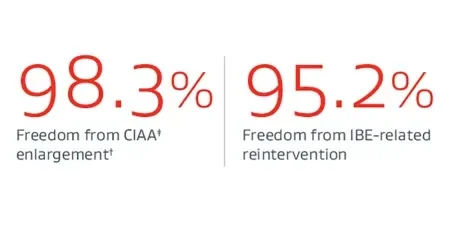
* U.S. IDE Clinical Trial. Sixty-three subjects with device implanted in initial cohort. Thirty-six patients have completed five-year follow-up.
† Core Lab reported assessment for patency, endoleak, migration and CIAA enlargement (>5mm). Denominator is number of subjects evaluated for primary effectiveness endpoint result with an evaluable result.
‡ On the side treated with the IBE.

* U.S. IDE Clinical Trial. Sixty-three subjects with device implanted in initial cohort. Thirty-six patients have completed five-year follow-up.
† Core Lab reported assessment for patency, endoleak, migration and CIAA enlargement (>5mm). Denominator is number of subjects evaluated for primary effectiveness endpoint result with an evaluable result.
‡ On the side treated with the IBE.
Why preservation matters
"If someone were to ask me why iliac preservation is necessary, there’s multiple reasons why that’s the case...the first and most important thing, it’s probably the best thing for the patients. Secondly, as Vascular Surgeons our whole job is about fixing and preserving blood vessels so if we can avoid sacrificing an important blood vessel, we should do that."
- Darren Schneider, M.D.
The recommended treatment1,2 to sustain quality of life
The GORE® EXCLUDER® Iliac Branch Endoprosthesis can be used with the GORE® EXCLUDER® AAA Endoprosthesis or GORE® EXCLUDER® Conformable AAA Endoprosthesis to create an off-the-shelf solution you can count on.
Learn more about our AAA devices.
- Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of Vascular Surgery 2018;67(1):2-77.e2.
- Moll FL, Powell JT, Fraedrich G, et al. European Society for Vascular Surgery. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. European Journal of Vascular & Endovascular Surgery 2011;41(Supplement 1):S1-S58.
Featured resources


Hypogastric Artery Treatment Algorithm
Algorithm developed by Advisory Board compromised of Vascular Surgeons when determining treatment of the hypogastric artery.
PDF, 173.59 KB
231054700-EN