CBAS Heparin Surface
Proven heparin bonding technology for lasting thromboresistance
Gore’s CBAS Heparin Surface, the proven heparin bonding technology for lasting thromboresistance, is used in many of our interventional and vascular surgery products. End-point covalent bonding keeps heparin anchored to the device, while the bioactive site remains free to interact with the blood to help prevent clotting.*
- Proven heparin availability: Performance-ready heparin active site1,4*
- Proven heparin bioactivity: Unmatched, persistent ability to take up antithrombin2,3*
- Proven lasting thromboresistance: Improved surface hemocompatibility resulting from heparin availability and bioactivity1,2,3,4,5*
*See full CBAS Heparin Surface references
Proprietary covalent end-point bonding
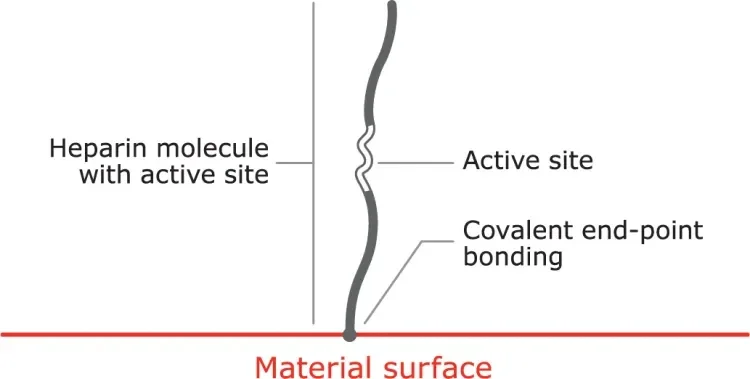
Covalent end-point bonding allows the heparin to extend into the bloodstream, keeping the active site bioavailable, unlike a non-permanent bond that can be washed away in the bloodstream.
- The anticoagulant function of heparin is dependent on the bioavailability of an active site within the molecule.
- Some methods of covalent heparin bonding damage and / or obstruct the active site, and hence destroy heparin’s anticoagulant activity.
- The CBAS® Heparin Surface consists of a proprietary covalent end-point bond that preserves the active site, thus retaining heparin’s anticoagulant activity.
Mechanism of action
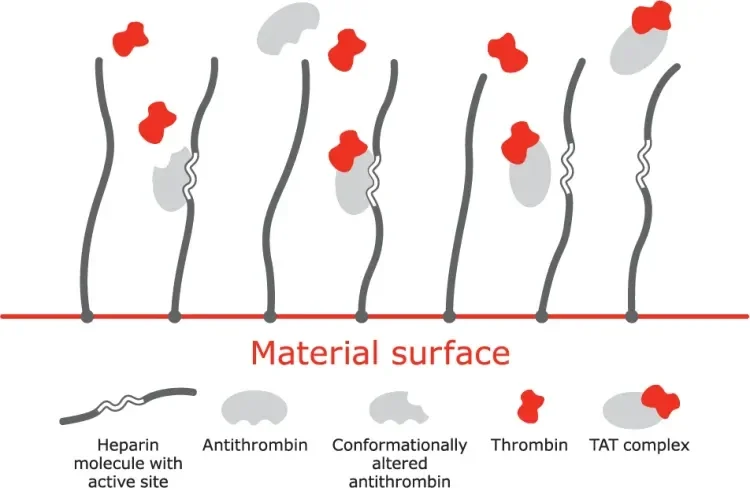
A. Bioactive site of the heparin molecule enables antithrombin to bind thrombin.
B. When antithrombin binds to thrombin, a neutral thrombin antithrombin (TAT) complex is formed.
C. Neutral TAT complex detaches from the heparin molecule. Active site becomes available to again bind antithrombin.
CBAS is a trademark of Carmeda AB, a wholly owned subsidiary of W. L. Gore & Associates, Inc.
Featured Gore products with CBAS Heparin Surface
50% reduction in the risk of graft occlusion compared to standard ePTFE in Critical Limb Ischemia (CLI) patients 1
Learn more >
1. Lindholt JS, Gottschalksen B, Johannesen N, et al. The Scandinavian Propaten® Trial – 1-year patency of PTFE vascular prostheses with heparin-bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses – a randomised clinical controlled multi-centre trial. European Journal of Vascular & Endovascular Surgery 2011;41(5):668-673.

Designed to expand to every demand, GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis offers proven procedural success and long-term outcomes through flexibility, strength, and accuracy to treat iliac occlusive disease.
LEARN MORE >

An Investigational Device Exemption (IDE) Clinical Study of the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface** in Japan demonstrated:
- 88% 12-month primary patency in long, complex SFA lesions (n = 103)1
- 21.8 cm average lesion length
- 65.7% chronic total occlusions (CTOs)
- 84.5% TASC II C&D lesions
Learn more >
** Heparin Bioactive Surface is synonymous with the CBAS Heparin Surface.
1. GORE® VIABAHN® Endoprosthesis Japan IDE Clinical Study demonstrated 12-month primary patency of 92% as defined by evidence of flow with no Target Lesion Revascularization (TLR). The same study demonstrated 88% 12-month primary patency when defined by PSVR of < 2.5 without a TLR.