Quality
Designed to fit the patient’s unique anatomy

Some call it conformability.
We call it one less thing for you to worry about.
Device conformability can reduce complications in patients with aortic disease requiring reintervention.1-3 That is why we design our aortic devices to adapt to the patient's unique anatomy.
At Gore, our portfolio of conformable stent grafts and innovative delivery systems allows us to support physicians in improving clinical outcomes in EVAR and TEVAR procedures, providing what matters to the patients: Long-lasting treatment.
Aortic endoprosthesis designed to respect the anatomy
Gore strives to redefine the standard of conformability. A device achieves conformability by combining radial force, device flexibility and spring-back force.
The GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System exhibited exceptional conformability based on results of spring-back analysis.
Elements of conformability
Low tendency
to spring back
Exceptional flexibility
while maintaining an open lumen
Designed to adapt
to the anatomy
Endovascular aortic repair devices to provide controlled conformability
Controlled conformability for each patient – building on the proven performance of the GORE® EXCLUDER® Device family, i.e. the conformable stent graft and innovative delivery system, offers new levels of control.
Most studied EVAR and TEVAR devices
We are a world leader in membrane technology and biomaterials and we are dedicated to creating durable quality that is built to last, to help give patients back their quality time. By undertaking comprehensive quality assessments, we develop trusted aortic solutions used in 675,000+ devices worldwide.* Our EVAR and TEVAR devices are the most studied worldwide†,‡ — a proven and durable option to treat patients.
Most studied
EVAR† and TEVAR‡ devices
Exceptional flexibility
while maintaining an open lumen
GORE® TAG® Conformable Thoracic Stent Graft
Anatomy through five-year follow-up3
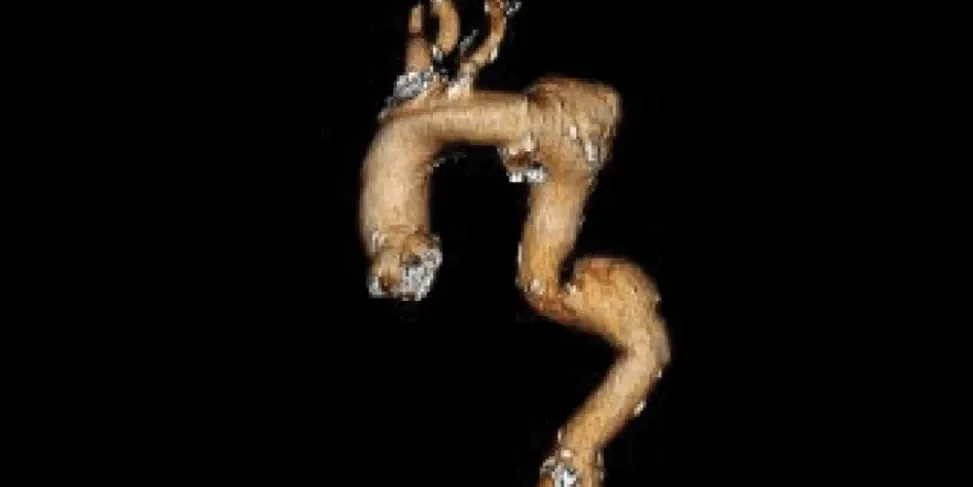
Pre-treatment
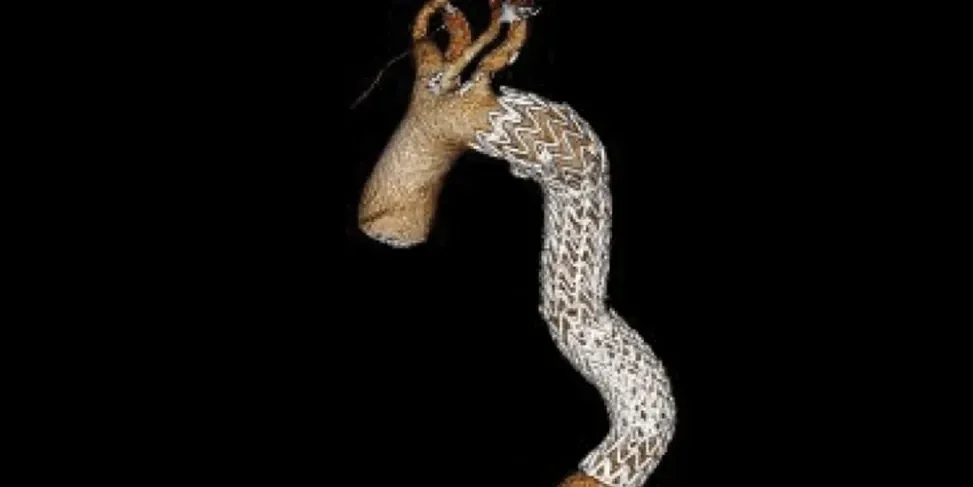
One year follow-up
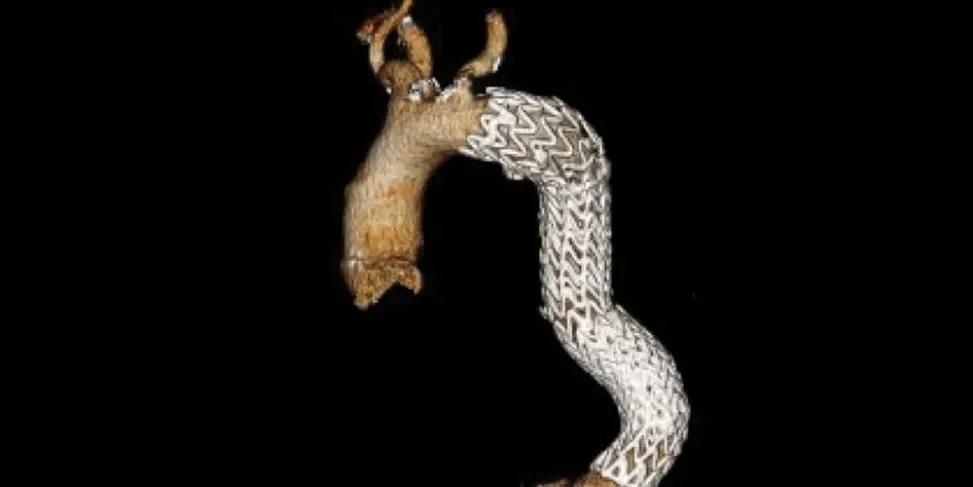
Two year follow-up
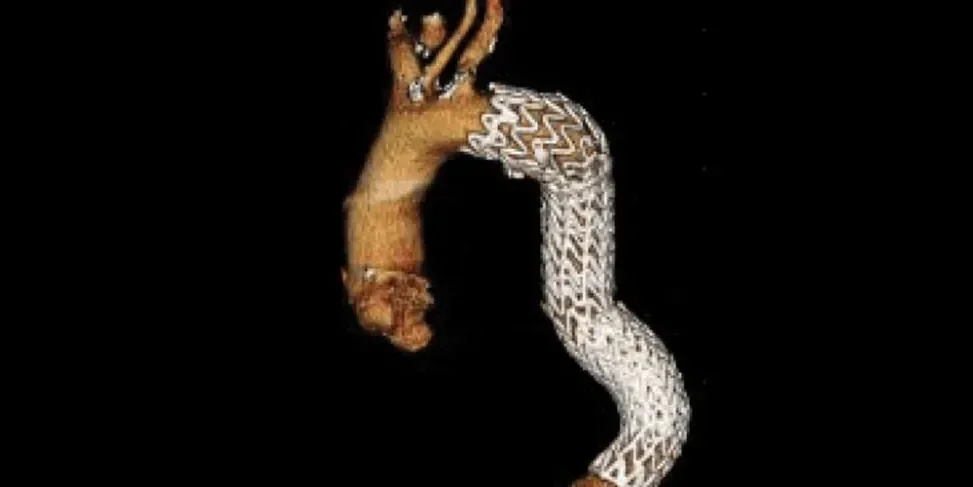
Three year follow-up
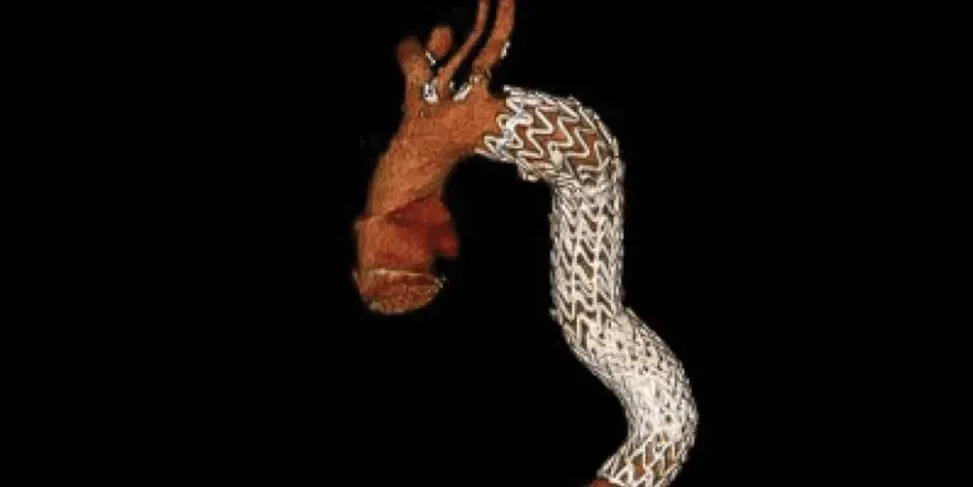
Four year follow-up

Five year follow-up
GORE® EXCLUDER®
Conformable AAA Endoprosthesis§
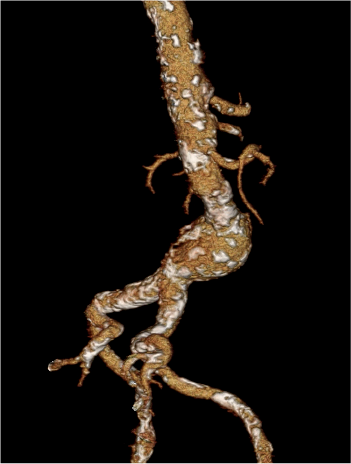
Pre-treatment

12-month follow-up
GORE® EXCLUDER®
Iliac Branch Endoprosthesis§
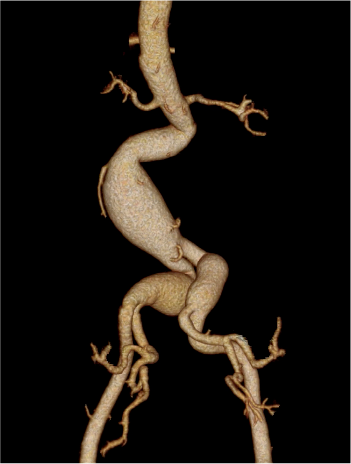
Pre-treatment
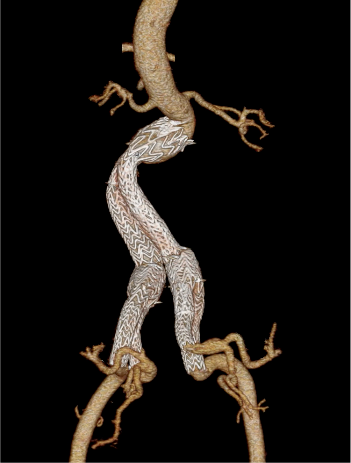
48-month follow-up
25 YEARS
Your Aortic Ally
A focus on the future, driven by a powerful past
At Gore, we have a strong heritage in material science leadership. Since the late 60s, Gore has been diving deep into material innovations and more for than 25 years, our devices are setting the standards for aortic care. But we have not stopped there. We continue to drive aortic innovations that will help physicians deliver the best possible care to their patients.
We’re your aortic ally.
Endografts designed to treat both straightforward and challenging anatomies
Our family of EVAR and TEVAR devices is designed for everyday practice and the treatment of highly challenging aortic pathologies. They all have one goal in common: Advancing aortic treatment capabilities.

GORE® TAG® Conformable Thoracic Stent Graft
New levels of control in the endovascular repair of aneurysms, transections and Type B dissections of the thoracic aorta

GORE® EXCLUDER® Conformable AAA Endoprosthesis
Deliver angulation, controlled conformability and precise placement in challenging proximal aortic necks
GORE® ACTIVE CONTROL System in EVAR and TEVAR
The GORE® ACTIVE CONTROL System is designed to increase the physician's confidence in deployment. It allows accurate device positioning and location refinement before full deployment and continuous blood flow throughout the entire procedure.
* Data on file, W. L. Gore & Associates, Inc; Flagstaff, AZ.
† Based on company-sponsored trials and registries shown on clinicaltrials.gov for currently available stent grafts.
‡ 10 FDA approved clinical studies.
§ Images from actual case study.
- Incidence and outcomes after infolding or collapse of thoracic stent grafts Kasirajan, Karthikeshwar et al. Journal of Vascular Surgery, Volume 55, Issue 3, 652 - 658.
- New C-TAG device overcome of compression events K. Kasirajan Journal of Cardiovascular Surgery, Volume 53, No. 2, 169-172.XX
- Donas KP, Inchingolo M, Cao P, et al; pELVIS Registry Collaborators. Secondary procedures following iliac branch device treatment of aneurysms involving the iliac bifurcation: The pELVIS Registry. Journal of Endovascular Therapy 2017;24(3):405-410.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
GORE® TAG® Conformable Thoracic Stent Graft
INDICATIONS FOR USE IN EUROPE: The GORE® TAG® Conformable Thoracic Stent Graft is indicated for endovascular repair of all lesions of the descending thoracic aorta, including isolated lesions, such as aneurysm and traumatic transection, and Type B dissections.
CONTRAINDICATIONS: The GORE® TAG® Conformable Thoracic Stent Graft is contraindicated in:
- Patients with known sensitivities or allergies to the device materials
- Patients with a systemic infection who may be at increased risk of endovascular graft infection
GORE® EXCLUDER® Conformable AAA Endoprosthesis
INDICATIONS FOR USE IN EUROPE: Trunk-Ipsilateral Leg Endoprosthesis and Contralateral Leg Endoprosthesis Components
The GORE® EXCLUDER® Conformable AAA Endoprosthesis is intended to exclude the aneurysm from the blood circulation in patients diagnosed with infrarenal abdominal aortic aneurysm (AAA) disease and who have appropriate anatomy as described below:
- Adequate iliac / femoral access
- Infrarenal aortic neck treatment diameter range of 16–32 mm
- A minimum aortic neck length of 10 mm when proximal aortic neck angulation is ≤ 60°
- A minimum aortic neck length of 15 mm when proximal aortic neck angulation is ≤ 90°
- Iliac artery treatment diameter range of 8–25 mm and iliac distal vessel seal zone length of at least 10 mm
Aortic Extender Endoprosthesis and Iliac Extender Endoprosthesis Components
The Aortic and Iliac Extender Endoprostheses are intended to be used after deployment of the GORE® EXCLUDER® Conformable AAA Endoprosthesis. These extensions are intended to be used when additional length and / or sealing for aneurysmal exclusion is desired.
CONTRAINDICATIONS: The GORE® EXCLUDER® Conformable AAA Endoprosthesis is contraindicated in:
- Patients with known sensitivities or allergies to the device materials.
- Patients with a systemic infection who may be at increased risk of endovascular graft infection.
GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE)
INDICATIONS FOR USE IN EUROPE: Iliac Branch and Internal Iliac Components
The GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE) is intended to isolate the common iliac artery from systemic blood flow and preserve blood flow in the external iliac and internal iliac arteries in patients with a common iliac or aortoiliac aneurysm, who have appropriate anatomy, including:
- Adequate iliac / femoral access
- Minimum common iliac diameter of 17 mm at the proximal implantation zone of the IBE
- External iliac artery treatment diameter range of 6.5–25 mm and seal zone length of at least 10 mm
- Internal iliac artery treatment diameter range of 6.5–13.5 mm and seal zone length of at least 10 mm
Trunk-Ipsilateral Leg and Contralateral Leg Endoprosthesis Components
The Trunk-Ipsilateral Leg and Contralateral Leg Endoprostheses are intended to provide proximal seal and fixation to the GORE® EXCLUDER® Iliac Branch Endoprosthesis following deployment of the GORE® EXCLUDER® Iliac Branch Endoprosthesis. For more information on the Trunk-Ipsilateral Leg and Contralateral Leg Endoprosthesis Component indications for use and deployment, see the GORE® EXCLUDER® AAA Endoprosthesis and GORE® EXCLUDER® Conformable Endoprosthesis Instructions for Use.
Aortic Extender Endoprosthesis and Iliac Extender Endoprosthesis Components
The Aortic and Iliac Extender Endoprostheses can be used after deployment of the GORE® EXCLUDER® Iliac Branch and AAA Endoprostheses. These extensions are used when additional length and / or sealing for aneurysmal exclusion is desired. For more information on Aortic Extender and Iliac Extender indications for use and deployment, see the GORE® EXCLUDER® AAA Endoprosthesis and GORE® EXCLUDER® Conformable Endoprosthesis Instructions for Use.
CONTRAINDICATIONS: The GORE® EXCLUDER® Iliac Branch Endoprosthesis is contraindicated in:
- Patients with known sensitivities or allergies to the device materials.
- Patients with a systemic infection who may be at increased risk of endovascular graft infection.