
GORE® CARDIOFORM Septal Occluder for PFO Closure
Gore REDUCE Clinical Study five-year results demonstrate PFO closure provides safe long-term prevention of recurrent stroke.
PFO closure with the GORE® CARDIOFORM Septal Occluder now has a well established benefit and robust safety profile over at least five years with a low risk of late complications. This is relevant for young stroke patients who undergo PFO closure to reduce the risk of a recurrence.
— Scott E. Kasner, M.D., National Principal Investigator for the REDUCE Study
Gore REDUCE Clinical Study five-year results demonstrate PFO closure provides safe long-term prevention of recurrent stroke.
- Published in the New England Journal of Medicine (NEJM), the REDUCE Study continues to show the largest reduction in recurrent ischemic stroke in all PFO shunt sizes over medical therapy alone.†,2
- Long-term results highlight GORE® CARDIOFORM Septal Occluder’s demonstrated record of patient safety and effective defect closure.
Robust trial design
The REDUCE Study evaluated whether PFO closure with a Gore device plus antiplatelet therapy reduces the risk of stroke compared to antiplatelet therapy alone.
- Prospective, randomized, multinational open-label trial.
- Standardized antiplatelet therapy for the medical arm. Trial designed to minimize medical therapy confounding.
- Prospective, 2-year MRI imaging.
- Inclusive of all PFO types.†,1
- 63 investigational sites in seven countries.
Key takeaways from long-term follow-up REDUCE study at five years:
- In carefully selected patients, PFO closure with the GORE® CARDIOFORM Septal Occluder device significantly reduced the risk of recurrent stroke compared to antiplatelet therapy alone over a five-year period.
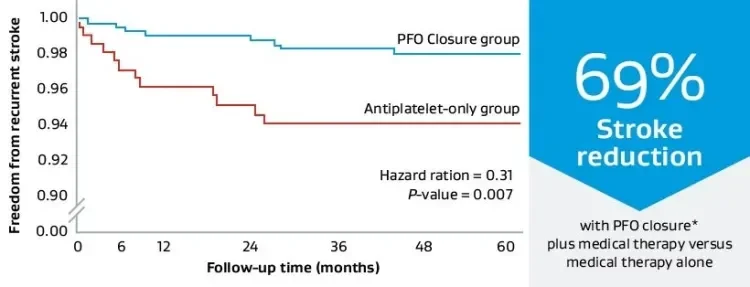
- 69% relative reduction in recurrent stroke versus medical management alone at five-year median follow-up.†,2
- Low risk of early device or procedure-related complications, none late.
- No reported cardiac erosions including through long-term follow-up.
- The majority of atrial fibrillation and atrial flutter cases were non-serious, early onset and resolved.2,3
- The benefit of closure persists long term with a low risk of late stroke, atrial fibrillation or other complications.
We are excited to publish the REDUCE Study long-term follow-up. These are important data as they confirm the procedure is safe with only one new episode of atrial fibrillation and no issues related to frame fractures, thrombus, embolization or erosion. Overall, the benefit of PFO closure persisted during late follow-up by lowering the risk of recurrent stroke with minimal risk for adverse events.
— John F. Rhodes, M.D., Medical University of South Carolina and U.S. Cardiology National Principal Investigator for the REDUCE Study
A leader in safety
Long-term results continue to demonstrate a legacy of patient safety with 2,069 patient years of data.1,2
Low risk of device or procedure-related serious adverse events (SAEs)
No new device or procedure-related SAEs at five years.
| REDUCE (median 5 years) |
|
|---|---|
| Device-related SAE | 6 (1.4%) |
| Procedure-related SAE | 11 (2.5%) |
The data presented below are from separate and independent clinical studies with different patient populations and interventions and thus are not a direct head-to-head comparison.
| REDUCE (median 5 years)2 |
RESPECT (median 5.9 years)4 |
|
|---|---|---|
| Serious device or procedure-related AFib | 2 (0.5%) | 2 (0.4%) |
| Subjects with post-implant AFib or flutter who had a recurrent stroke | 1 (0.2%) | 1 (0.2%) |
No reports of erosion
| REDUCE (median 5 years)2 |
|
|---|---|
| Cardiac erosion | 0 |
Trusted closure performance
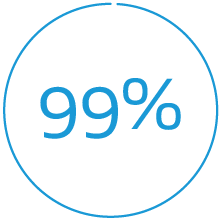
Effective closure across PFO anatomies at 24 months**,††
Clinically proven secondary stroke prevention
The REDUCE Study continues to show the largest reduction in recurrent ischemic stroke in all PFO shunt sizes over medical management alone.†,2
Stroke reduction data
Ischemic stroke reduction relative to medical management.
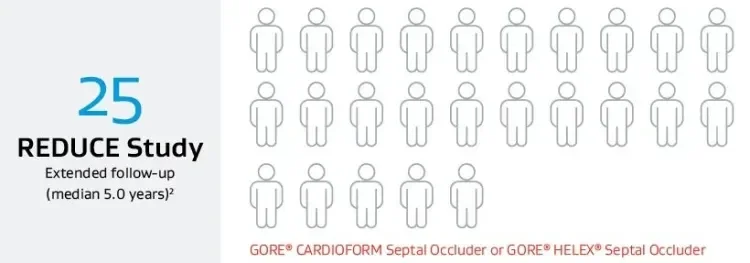
Compelling real-world results
Number of patients needed to treat to prevent one recurrent ischemic stroke at five years.
See the New England Journal of Medicine article
3.2 years (original follow-up)
5.9 years (extended follow-up)
* In patients with a PFO and history of cryptogenic stroke.
† The REDUCE Study determined safety and efficacy of PFO closure with the GORE® CARDIOFORM Septal Occluder or GORE® HELEX® Septal Occluder plus antiplatelet medical management compared to antiplatelet medical management alone in patients with a PFO and history of cryptogenic stroke. All PFO anatomies were eligible for inclusion into this study within indicated sizing parameters of the Instructions for Use.
‡ REDUCE is the only U.S. IDE trial that achieved its primary endpoint, and over five years showed a significant reduction in recurrent ischemic stroke across PFO anatomies over medical therapy alone.
§ Cryptogenic diagnosed as: No stenosis > 50 percent or ulcerated plaque in relevant intra- or extra-cranial vessels, no atrial fibrillation or high-risk source of cardioembolism, non-lacunar (based on neuroimaging), no evidence of hypercoagulable disorder, no other known cause of stroke.
II PFO confirmed by transesophageal echocardiography (TEE / TOE) with bubble study demonstrating right-to-left shunt at rest or during Valsalva maneuver. Patients with PFO eligible regardless of shunt size or presence of atrial septal aneurysm.
** Effective closure defined as freedom from large shunt > 25 bubbles as detected by transthoracic echocardiography adjudicated by Echo Core Lab.
†† Data on file 2020; W. L. Gore & Associates, Inc.; Flagstaff, AZ
- Søndergaard L, Kasner SE, Rhodes JF, et al.; Gore REDUCE Study Investigators. PFO closure or antiplatelet therapy for cryptogenic stroke. New England Journal of Medicine 2017;377(11):1033-1042.
- Kasner SE, Rhodes JF, Andersen G; Gore REDUCE Clinical Study Investigators. Five-year outcomes of PFO closure or antiplatelet therapy for cryptogenic stroke. New England Journal of Medicine 2021;384(10):970-971.
- Kasner, SE. Long-term outcomes with patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. Presented virtually on November 7, 2020, at European Stroke Organisation & World Stroke Organisation conference.
- Sayer JL, Carroll JD, Thaler DE, et al.; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. New England Journal of Medicine 2017;377(11):1022-1032.
Products listed may not be available in all markets.
ABBOTT and AMPLATZER are trademarks of Abbott Laboratories.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231204050-EN