Earlier TIPS
Earlier TIPS procedure for ascites
Earlier TIPS shows significant improvement in outcomes compared to large-volume paracenteses and albumin infusion (LVP+A) at one year.1
HIGHER
transplant-free survival at one year
93% TIPS vs. 52% LVP+A (P = .003)
LESS
recurrence of ascites
32 TIPS vs. 320 LVP+A (P < .001) total number of paracenteses (TIPS n = 29, LVP+A n = 33)
FEWER
complications
0% TIPS vs. 18% LVP+A (P = .01) portal hypertension-related bleeding and hernia-related complications
FEWER
hospitalization days
Patients treated with TIPS averaged 17 days vs. 35 for those treated with LVP+A
NO DIFFERENCE
in hepatic encephalopathy
65% TIPS vs. 65% LVP+A (P = .868) probability of remaining free of hepatic encephalopathy
Earlier TIPS procedure for variceal bleeding
Evidence shows that early TIPS can significantly improve outcomes in select liver disease patients.*,3
HIGHER survival
in Child-Pugh C patients at one year
78% TIPS vs. 53% pharmacotherapy+EBL (P = .002)
GREATER freedom
from rebleeding and treatment failure
92% TIPS vs 74% pharmacotherapy+EBL (P = .017) freedom from failure to control bleeding or prevent rebleeding in Child-Plug B+AB (active bleeding) and C patients
LESS frequent
de novo ascites or worsening of previous ascites
9.1% TIPS vs. 47.6% pharmacotherapy+EBL (P < .001) in Child-Pugh B+AB and C patients
NO DIFFERENCE
in hepatic encephalopathy
42.4% TIPS vs. 37.7% pharmacotherapy+EBL (P = .683) experienced hepatic encephalopathy
Some patients with portal hypertension never experience esophageal variceal bleeding or are able to control their symptoms by taking medication. However, for other patients, medication and minimally invasive therapy fail to stop a bleeding episode or prevent recurrent bleeding episodes. For these patients with a high-risk of rebleeding, multiple studies have shown that the TIPS procedure is more effective at controlling and preventing esophageal variceal bleeding than continued treatment with medication and minimally invasive therapy.3-5
These studies have also shown that the TIPS procedure can increase survival in select patients.3 In several of these studies, the risk of hepatic encephalopathy was similar in patients who underwent the TIPS procedure and patients that continued with medication and minimally invasive therapy.3-5 The timing of when your TIPS procedure is performed is important, and the studies described focus on “early” TIPS where patients undergo a TIPS procedure within 72 hours of an acute esophageal bleeding episode. To determine whether the TIPS procedure can help control your esophageal variceal bleeding and increase your survival, ask your doctor if you are a good candidate for the TIPS procedure.
Early TIPS improves survival and control of bleeding compared to repeat endoscopic procedures with medical management.
| Trial or study | Survival at one year | Prevention of rebleeding | Incidence of hepatic encephalopathy compared to pharmacotherapy combined with endoscopic therapy |
|---|---|---|---|
| Randomized Control Trial (RCT)4 | 86%† | 97%§ | No significant difference |
| Post-RCT Surveillance Study5 | 86%† | 93%II | No significant difference |
| Observational Study3 | 78%† | 92%¶ | No significant difference |
Early TIPS procedure with controlled expansion endoprosthesis
GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion combines the legacy of proven patency6 with diameter control to reach a targeted portal pressure gradient. The innovations include:
- Control the diameter — Designed to reach a targeted portal pressure gradient
- Lasting diameter control** — Size and set the diameter to stay
- Engineered for flexibility — Conformability to tortuous anatomy
GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion specifications
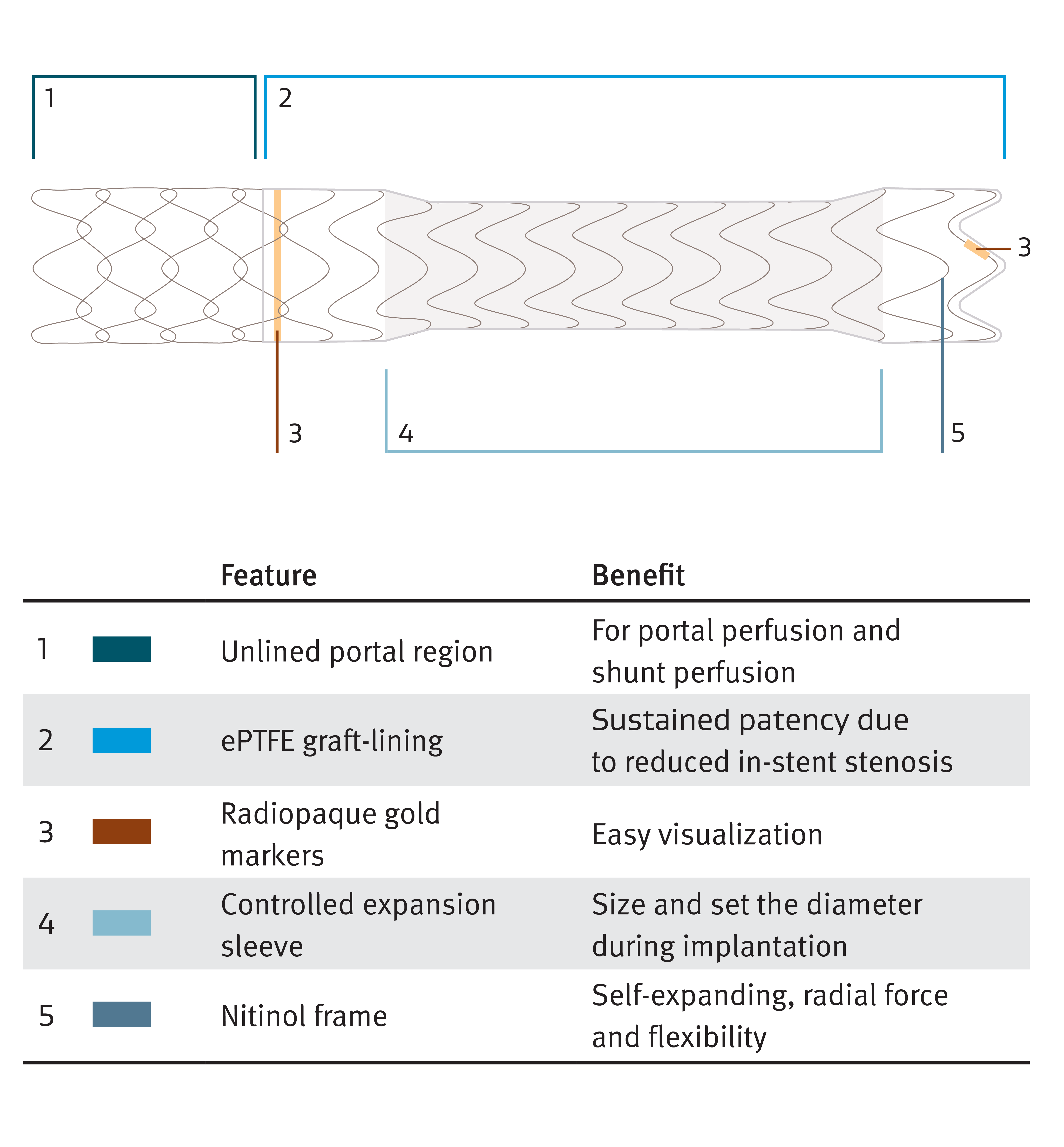
The GORE® VIATORR® TIPS Device
For your patients with portal hypertension, the GORE® VIATORR® TIPS Endoprosthesis maintains significantly increased patency compared to bare metal stent alternatives. Highly effective in lowering portal pressure gradients in patients with refractory ascites and variceal bleeding, the GORE® VIATORR® TIPS Endoprosthesis offers an effective treatment option for patients over a longer period of time.†† The GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion allows doctors to adjust the diameter of the device during implantation based on the patient’s needs and portal pressure.
Two clinical trials were conducted in the United States and one randomized controlled trial in Europe to evaluate the GORE® VIATORR® TIPS Endoprosthesis and the BOSTON SCIENTIFIC WALLSTENT Endoprosthesis Stent for use in de novo TIPS and TIPS revision. These studies demonstrated that primary patency of the GORE® VIATORR® Device group was superior to that of the BOSTON SCIENTIFIC WALLSTENT Device group (P < .001) at six months, with no significant differences in mortality or risk of encephalopathy.††
Gore products referenced within are used within their FDA approved/cleared indications or are under investigation for the uses referenced within. Gore does not have knowledge of the indications and FDA approval/clearance status of non-Gore products. Gore makes no representations as to the surgical techniques, medical conditions or other factors that may be described in the article. The reader is advised to contact the manufacturer for current and accurate information.
This information is intended for education and awareness only. Patients should consult their physician for information on the risks associated with the devices and surgical procedures discussed in this website. All surgical procedures carry potential health risks. Not all patients will be candidates for treatment with these devices, and individual outcomes may vary.
Always follow physician advice on your post-surgery care and recovery.
* Early TIPS (n = 66) compared to pharmacotherapy+endoscopic band ligation (EBL) (n = 605). Child-Pugh C patients with scores < 14.
† For a combined group of patients with Child-Pugh C (CP-C) score ≤ 13 or Child-Pugh B with active bleeding (CP-B + AB) at diagnostic endoscopy.
‡ For Child-Pugh C patients with scores ≤ 13.
§ One-year actuarial probability of remaining free of failure to control bleeding and of variceal rebleeding.
II Primary study end point is composite outcome of failure to control acute bleeding or to prevent clinically significant variceal rebleeding. Data is presented as percentage that did not present the composite outcome.
¶ Composite end point of failure to control acute bleeding, early rebleeding, and late rebleeding. Data as presented is for CP-B + AB and CP-C patients combined.
** Based on benchtop data on file. Less than 0.25 mm increase in diameter (diameter expansion) demonstrated by a simulated 10 year period at physiologic portal pressures.
†† On file with Gore PMA P040027.
- Bureau C, Thabut D, Oberti D, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152(1):157-163. http://www.sciencedirect.com/science/article/pii/S0016508516351101
- Piecha F, Radunski UK, Ozga AK, et al. Ascites control by TIPS is more successful in patients with a lower paracentesis frequency and is associated with improved survival. JHEP Reports 2019;1(2):90-98. https://www.sciencedirect.com/science/article/pii/S258955591930031X
- Hernández-Gea V, Procopet B, Giráldez Á, et al. International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS improves outcome in high-risk variceal bleeding: an observational study. Hepatology 2019;69(1):282-293. https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.30182
- García-Pagán JC, Caca K, Bureau K, et al. Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. New England Journal of Medicine 2010;362(25):2370-2379. https://www.nejm.org/doi/full/10.1056/NEJMoa0910102
- Garcia-Pagán JC, Di Pascoli M, Caca K, et al. Use of early-TIPS for high-risk variceal bleeding. Results of a post-RCT surveillance study. Journal of Hepatology 2013;58(1):45-50.
- Bureau C, Pagan JCG, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long term results of a randomized multicentre study. Liver International 2007;27(6):742-747.
BOSTON SCIENTIFIC and WALLSTENT are trademarks of Boston Scientific Corporation.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion is indicated for use in the treatment of portal hypertension and its complications such as variceal bleeding and refractory ascites.
CONTRAINDICATIONS: There are no known contraindications for this device.