Advancing endovascular options: New endovascular devices and insights to extend aortic treatment to more patients
ADVANCING ENDOVASCULAR OPTIONS
Sponsored by Gore & Associates
LSA preservation during TEVAR: Insights from the International GREAT Registry
An overview of our experience with the Conformable GORE® TAG® Thoracic Endoprosthesis.
Dennis R. Gable, MD
William T. Brinkman, MD
The use and technologic advances of thoracic aortic endografts continue to increase and provide a less invasive option to treat a variety of thoracic aortic pathologies including aneurysm, dissection, intramural hematoma, traumatic transection, fistula, and penetrating ulcer. Coverage of the left subclavian artery (LSA) allows for increased utilization of thoracic endovascular aortic repair (TEVAR) in patients who otherwise would have anatomic limitations that may prevent endovascular repair due to a short proximal landing zone.1
By covering the LSA, surgeons may gain at least 2 cm of proximal landing zone in which to place the stent graft. In fact, LSA coverage is performed in up to 40% of TEVAR procedures to ensure an adequate seal in the proximal landing zone.2 In most cases, coverage of the LSA without revascularization is well tolerated by patients, and endoleak is prevented due to thrombosis of the proximal 1 cm of LSA coming off the arch. However, the risk for limb ischemia as well as a posterior circulation stroke and an increased risk of paraplegia due to reduced collateral spinal perfusion pathways remain major concerns.
Despite the frequency with which the LSA is covered, it remains controversial whether revascularization of the LSA should be performed and, if so, which method of revascularization is most effective.3 There is support for routine LSA revascularization based on findings from the EUROSTAR registry, which reported a significantly higher incidence of spinal cord ischemia or stroke (8.4%) in patients who did not undergo LSA revascularization compared to patients who were revascularized (0%; P = .49).4
In certain cases, LSA revascularization is required; absolute indications include patients with a previous patent left internal mammary artery–left anterior descending artery bypass or a dominant left vertebral artery. The presence of a left upper extremity dialysis graft is a relative contraindication to LSA coverage without revascularization except in emergent situations. Revascularization should be strongly considered in patients with additional risk factors for spinal cord ischemia such as those who have previously undergone open infrarenal surgical revascularization or prior endovascular aneurysm repair (EVAR) of the infrarenal aorta. LSA revascularization should also be considered for cases where stent graft coverage is planned for a long segment of the thoracic aorta, as the LSA is a key source of blood flow to the upper spinal cord. Atresia or occlusion of the right vertebral artery or stenosis/occlusion of the hypogastric arteries are both indications for revascularization to assist in avoiding spinal cord ischemia.
The 2010 Society for Vascular Surgery guidelines recommend preoperative revascularization of the LSA when possible in nonemergent cases, although this recommendation was made based on a low level of evidence.5 The published guidelines outline the following recommendations based on the best available evidence, as assessed by the grading of recommendations assessment, development, and evaluation (GRADE) system5:
- In patients who need elective TEVAR where achievement of a proximal seal necessitates coverage of the LSA, routine preoperative revascularization is suggested (GRADE 2, level C)
- In select patients who have anatomy that compromises perfusion to critical organs, routine preoperative LSA revascularization is strongly recommended (GRADE 1, level C)
- In patients who need urgent TEVAR for life-threatening acute aortic syndromes where achievement of a proximal seal necessitates coverage of the LSA, LSA revascularization should be individualized and addressed expectantly on the basis of anatomy, urgency, and availability of surgical expertise (GRADE 2, level C)
A systematic review and meta-analysis of peer-reviewed studies demonstrated that the results of studies investigating intentional LSA coverage during TEVAR with versus without revascularization were mixed. Some studies demonstrated increased risks for stroke, extremity ischemia, and/or spinal cord ischemia, whereas others found no increased risk for postoperative morbidity.1 After combining the results of these 51 studies, all of which had a retrospective observational design, the analysis found that LSA coverage without revascularization was associated with a significantly increased risk of extremity and vertebrobasilar ischemia and a trend toward increased paraplegia and anterior circulation stroke. In exchange, revascularization was associated with a 4% chance of phrenic nerve injury.
Even in the setting of revascularization of the LSA prior to TEVAR, cerebrovascular flow patterns in the left vertebral artery can be affected. In a cohort of 74 patients who underwent carotid duplex studies before and after TEVAR with LSA bypass, decreased postoperative antegrade flow was seen in the left vertebral artery, with a concomitant decrease in peak systolic velocity.6 In contrast, the peak systolic velocity increased in the right vertebral artery and right internal carotid artery. If and how these hemodynamic changes impact clinical events after TEVAR are unknown. Notably, these data were not compared with findings from patients who did not undergo LSA revascularization prior to TEVAR. The authors hypothesized that the retrograde flow in the LSA established with a carotid subclavian bypass may play a role. Larger studies are needed to determine the significance of this finding in relation to postoperative neurologic events in patients undergoing LSA revascularization before TEVAR. Ultimately, the question of when and whether to revascularize the LSA before or during TEVAR remains unanswered, with no available level 1 evidence.
OPTIONS FOR REVASCULARIZATION
Carotid-Subclavian Artery Bypass
Carotid-subclavian artery bypass is a procedure that has shown exceptional results.7,8 It is the preferred technique for patients with a left vertebral artery originating very proximally off of the LSA, as extensive proximal dissection is not required for completion. The procedure is typically performed through a supraclavicular incision beginning over the clavicular head of the sternocleidomastoid muscle and extending laterally approximately 3 to 4 cm. Dissection is carried down to the level of the jugular vein, which is reflected medially, allowing access to the common carotid artery (CCA). The vagus nerve and the sympathetic chain are posteriorly identified and preserved. The scalene fat pad is mobilized off of the anterior scalene muscle. The phrenic nerve runs along the surface of the anterior scalene muscle and should be identified to avoid injury. Care must be taken to avoid the thoracic duct, which runs posteriorly to the left carotid artery and internal jugular vein. If the thoracic duct is encountered in the dissection, it should be ligated to avoid postoperative lymphocele.
A prosthetic graft, as compared to an autologous venous conduit, is considered the conduit of choice in carotid-subclavian bypass. In 1986, Ziomek et al showed that 5-year patency rates were superior with prosthetic grafts compared to autogenous vein grafts (94.1% vs 58.3%, respectively; P < .01).7 These results were supported by Law et al who demonstrated 5-year patency rates of 95.2% for polytetrafluoroethylene grafts, 83.9% with Dacron grafts, and 64.8% with saphenous vein grafts.8
Carotid-Subclavian Artery Transposition
Carotid-subclavian artery transposition allows revascularization of the LSA without the use of any prosthetic graft material. The disadvantage to subclavian transposition is that it requires more extensive dissection to gain proximal control and mobilize a sufficient length of the subclavian artery to allow tension-free anastomosis to the carotid artery. Because of these limitations, transposition is contraindicated in patients with an early origin of the vertebral artery and in those with a patent left internal mammary artery–coronary artery bypass graft. Transposition is typically performed through a short medial transverse incision made just above the level of the clavicle. Dissection is carried down between the heads of the sternocleidomastoid muscle, the omohyoid muscle is divided, and the jugular vein is retracted laterally. On the left, the thoracic duct should be identified and ligated. Dissection of the CCA is performed, ensuring preservation of the vagus nerve. The vertebral vein is divided to gain access to the subclavian artery, which is controlled as proximally as possible and must be done proximal to the vertebral artery to preserve posterior cerebral circulation. The subclavian artery is then divided to perform an end-to-side anastomosis to the CCA. Transposition has excellent outcomes with good patency rates. Duran et al reported a patency rate of 96.3% at 53.8 months in 126 patients who underwent transposition.9
THE GREAT REGISTRY
The Global Registry for Endovascular Aortic Treatment (GREAT) is an industry-sponsored initiative designed to tabulate real-world patient outcomes and device performance with up to 10-year follow-up in patients treated with aortic endografts (EVAR or TEVAR) developed by a single device company (Gore & Associates).10 The prospective registry includes data from 114 centers (high and low volume, academic and nonacademic) using Gore’s commercially available aortic endograft products in the United States, Europe, Australia/New Zealand, and Brazil. The registry recently completed enrollment of over 5,000 patients.10 We sought to evaluate the documented experience with the Conformable GORE® TAG® Thoracic Endoprosthesis regarding LSA coverage and revascularization strategies (Figure 1). All patients undergoing TEVAR using the device and with a zone 2 proximal landing and LSA-only involvement were identified. Cases with a chimney procedure, thoracoabdominal aneurysm, type A dissection, or incomplete procedural information were excluded.
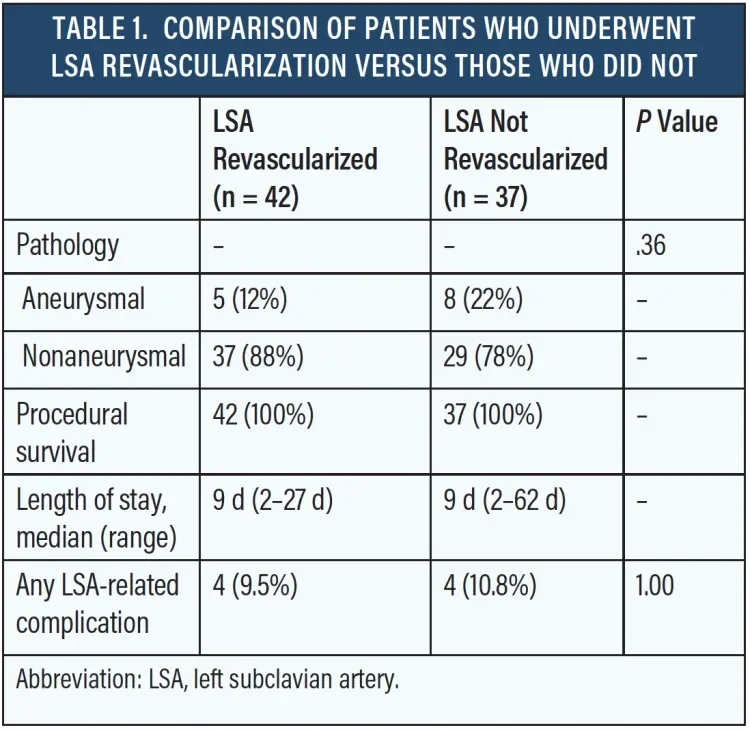
A total of 79 patients met the study criteria. Of these, 42 patients (53%) underwent LSA revascularization and 37 (47%) did not. Eight (10%) postoperative complications related to coverage or revascularization of the LSA were reported. Among the patients who were revascularized, complications included paraplegia (n = 1), spinal cord ischemia manifesting as bilateral lower extremity weakness and requiring lumbar drainage (n = 1), recurrent laryngeal nerve injury during surgical debranching (n = 1), and one type Ia endoleak requiring embolization of the stump of the LSA (n = 1). Patients who did not undergo LSA revascularization had the following postoperative complications: subclavian steal syndrome requiring left carotid–to–left subclavian bypass (n = 2), stroke (n = 1), and increased expansion of descending thoracic aneurysms requiring explantation and graft replacement (n = 1). Procedural survival was 100% in both groups, and total hospital length of stay did not differ between treatment strategies (Table 1).
DISCUSSION
The worldwide experience with the Conformable GORE TAG Thoracic Endoprosthesis with zone 2 landing demonstrates that a variety of aortic pathologies can safely be treated via TEVAR with this device. Interestingly, the registry demonstrates that surgeons elected to revascularize the LSA in roughly only half of instances of zone 2 proximal landing zones. This finding reflects the ongoing debate regarding the advantages and disadvantages of LSA revascularization in the absence of strong evidence-based guidelines to direct practice patterns.
Also of note were the roughly equal rates of perioperative complications (approximately 10%) related to decision in both groups. Although the incidences of complications were similar, the morbidities experienced by patients in each group were naturally different. For example, two patients undergoing revascularization were not spared complications related to spinal cord ischemia despite the proposed advantage of revascularization to reduce this complication, whereas no cases of spinal cord ischemia were reported in the nonrevascularized group. Another revascularized patient suffered recurrent laryngeal nerve injury during surgical debranching, a risk that patients are not exposed to if they are not revascularized. Meanwhile, nonrevascularized patients were at risk for stroke and extremity ischemia, neither of which were reported in the revascularized group.
Although the sample size and event rates were not sufficient for statistical analysis to compare LSA revascularization versus nonrevascularization, these findings reflect the experience documented in peer-reviewed literature over the last decade. For now, the GREAT registry demonstrates the safety of the Conformable GORE TAG Thoracic Endoprosthesis for TEVAR requiring zone 2 landing. Optimal LSA management strategies can only be understood through continued experience with, and evaluation of, this endograft and other thoracic aortic endografts. One of the new technologic advances that may play a role in decreasing the risk of revascularization while allowing for LSA perfusion through an endovascular approach is the new GORE® TAG® Thoracic Branch Endoprosthesis (TBE)* (Figure 2). Pivotal studies are currently ongoing, and outcomes from this study will be available in the near future. These results may offer new options and improve the outcomes for this patient population.
- Rizvi AZ, Murad MH, Fairman RM, et al. The effect of left subclavian artery coverage on morbidity and mortality in patients undergoing endovascular thoracic aortic interventions: a systematic review and meta-analysis. J Vasc Surg. 2009;50:1159-1169.
- Feezor RJ, Martin TD, Hess PG, et al. Risk factors for perioperative stroke during thoracic endovascular aortic repairs (TEVAR). J Endovasc Ther. 2007;14:568-573.
- Bradshaw RJ, Ahanchi SS, Powell O, et al. Left subclavian artery revascularization in zone 2 thoracic endovascular aortic repair is associated with lower stroke risk across all aortic diseases. J Vasc Surg. 2017;65:1270-1279.
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: incidence and risk factors. A study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg. 2007;46:1103-1110.
- Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg. 2009;50:1155-1158.
- Kamman AV, Eliason JL, Williams DM, et al. Impact of left subclavian artery revascularization before thoracic endovascular aortic repair on postoperative cerebrovascular hemodynamics. Ann Vasc Surg. 2018;46:307-313.
- Ziomek S, Quiñones-Baldrich WJ, Busuttil RW, et al. The superiority of synthetic arterial grafts over autologous veins in carotid-subclavian bypass. J Vasc Surg. 1986;3:140-145.
- Law MM, Colburn MD, Moore WS, et al. Carotid-subclavian bypass for brachiocephalic occlusive disease. Choice of conduit and long-term follow-up. Stroke. 1995;26:1565-1571.
- Duran M, Grotemeyer D, Danch MA, et al. Subclavian carotid transposition: immediate and long-term outcomes of 126 surgical reconstructions. Ann Vasc Surg. 2015;29:397-403.
- Loa J, Dubenec S, Cao P, et al. The Gore Global Registry for Endovascular Aortic Treatment: objectives and design. Ann Vasc Surg. 2016;31:70-76.
Products listed may not be available in all markets.
See all products by region:
Dennis R. Gable, MD
Department of Vascular and Endovascular Surgery
The Heart Hospital Baylor Plano
Plano, Texas
Disclosures: Consultant to and receives research support and honoraria from Gore & Associates and Medtronic.
William T. Brinkman, MD
Department of Cardiothoracic Surgery
The Heart Hospital Baylor Plano
Plano, Texas
Disclosures: Consultant to and trainor for Edwards Lifesciences and Medtronic.