Advancing endovascular options: New endovascular devices and insights to extend aortic treatment to more patients
ADVANCING ENDOVASCULAR OPTIONS
Sponsored by Gore & Associates
Optimizing TEVAR Outcomes in acute and subacute Type B Aortic dissections
Procedural planning and intraoperative techniques to increase the likelihood of success.
Bruce Tjaden, MD
Ali Azizzadeh, MD, FACS
The use of thoracic endovascular aortic repair (TEVAR) for the treatment of acute and subacute type B aortic dissections (a/sTBADs) can challenge even the most experienced vascular surgeon. Deciding which patients to treat, selecting the appropriate endograft, sizing the device correctly, and minimizing technical difficulties and complications are all critical steps and require careful thought and planning. In this article, we provide a summary of considerations and best practices to optimize outcomes in TEVAR for a/sTBAD.
DEFINING AND TRIAGING ACUTE AND SUBACUTE TYPE B AORTIC DISSECTIONS
For the purposes of this discussion, we will define acute type B aortic dissections (aTBADs) as those in which the onset of symptoms occurred < 14 days before presentation. Subacute type B aortic dissections (sTBADs) are those in which the onset of symptoms occurred between 14 to 90 days before presentation, and chronic dissections are those in which the dissection is older than 90 days. Malperfusion is defined as clinical end-organ ischemia affecting the brain, spinal cord, viscera, kidneys, and/or lower extremities.
The first clinical question that becomes important in the care of patients with a/sTBAD is who to treat. Patients with malperfusion mandate expeditious treatment—with each minute of critical malperfusion, the potential for poor outcomes and death increases (Figure 1). Similarly, in the rare patients who present with acute dissection complicated by rupture, emergency treatment to seal the site of extravasation is required.
However, in patients with uncomplicated a/sTBAD, the benefits of TEVAR must be balanced against the risks of perioperative complications. In order to choose the best therapy for an individual patient, it is important to know which patients are more likely to fail on medical therapy alone. Our large institutional series of uncomplicated TBADs revealed that patients with a total aortic diameter of > 44 mm at any location were more likely to require intervention and had an increased risk of mortality compared to those with a smaller aortic diameter (< 44 mm). Similarly, patients with a false lumen (FL) diameter > 22 mm had decreased intervention-free survival. Patients older than 60 years had an increased risk of mortality when compared to younger patients.1 Furthermore, in our clinical experience, a/sTBAD patients with “borderline” malperfusion symptoms such as intermittent nausea and vomiting, prolonged ileus, or fluctuating pulse exams seem more likely to progress to frank malperfusion requiring urgent TEVAR. Another high-risk subgroup is patients with refractory pain and hypertension despite medical therapy. Studies have shown that these patients do significantly worse compared to those who have no pain or refractory hypertension.2 Patients with these high-risk features should be carefully considered for TEVAR, sooner rather than later, to avoid the risks of delayed complications.
Next, a decision must be made as to the timing of TEVAR. There is evidence that the risks of perioperative complications such as rupture, retrograde type A dissection (RTAD), and mortality are decreased if TEVAR is delayed until the subacute time frame.3,4 For this reason, it is our practice that patients with uncomplicated a/sTBAD and high-risk features are treated medically for 2 to 6 weeks and then undergo elective endovascular repair.
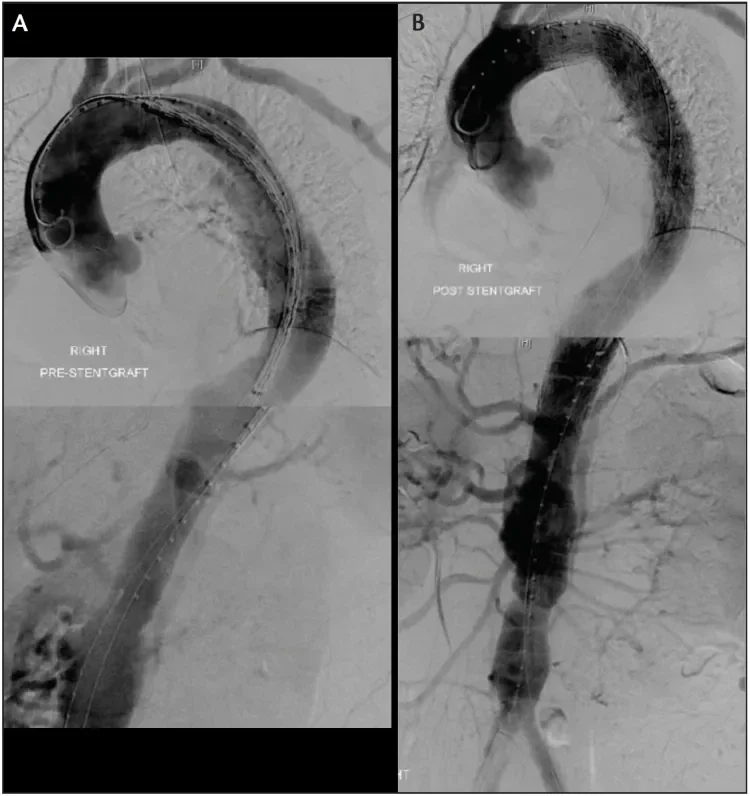
Figure 1. A 56-year-old man presented with an acute complicated TBAD with visceral and left lower extremity malperfusion. He was treated with two Conformable GORE® TAG® Devices. The pre-TEVAR imaging demonstrates poor filling of the mesenteric vessels (A), whereas the post-TEVAR imaging shows brisk filling throughout the renovisceral segment as well as both iliac arteries after TEVAR (B).
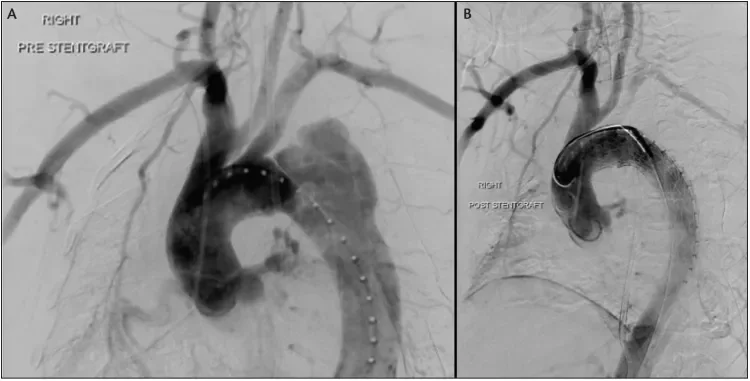
Figure 2. A 55-year-old woman initially presented with acute uncomplicated TBAD. She was managed medically but developed renal malperfusion during the subacute phase, mandating repair. During TEVAR, angiography revealed interval development of a contained rupture (A). IVUS confirmed that the dissection extended into the distal aortic arch. The operative plan was modified, and a Conformable GORE® TAG® Device was deployed with intentional LSA coverage. A completion angiogram revealed adequate filling of the LSA through retrograde vertebral flow, exclusion of the diseased aorta, and brisk distal TL filling (B). She was followed expectantly and did not require LSA revascularization.
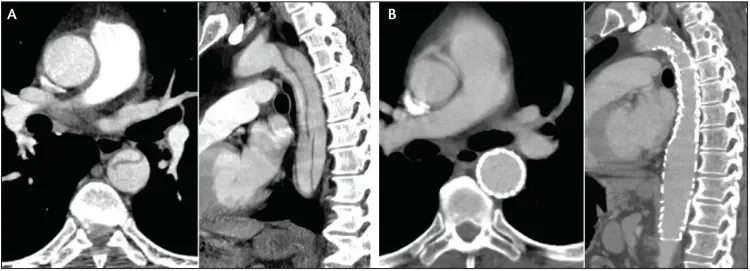
Figure 3. A 75-year-old man presented with an acute complicated TBAD with a large fenestration in the mid descending thoracic aorta (A). TEVAR resulted in exclusion of the diseased aorta, with excellent aortic remodeling seen at 6 months and no endoleak (B).
PLANNING ENDOVASCULAR REPAIR
Landing Zones
There are multiple goals of TEVAR for aortic dissection: to close the proximal entry tear, alleviate malperfusion, prevent or treat rupture, pressurize the true lumen (TL), and induce FL thrombosis. However, the unique anatomy of each dissection often makes this a complex task. The proximal landing zone must be in an area of normal aorta that is unaffected by dissection. Since the proximal tear is often just distal to the left subclavian artery (LSA), we most often plan on landing the endograft at the distal edge of the LSA. Occasionally, we may position the leading edge of the device fabric partially covering the ostia of the LSA to gain additional seal zone and to ensure that the entry tear is covered. It is not uncommon for a small component of intramural hematoma (IMH) to extend along the outer curvature of the aorta proximally in a retrograde fashion, even though there is no overt dissection flap in that area. We generally do not consider the presence of limited retrograde IMH to be a contraindication to landing in that region, as long as the most proximal part of the device is in nondissected aorta.
In approximately 40% of cases, coverage of the LSA is required to seal the proximal entry tear. In the emergent setting, we typically cover the LSA without revascularization unless hard indications for revascularization are present (Figure 2). These include previous coronary artery bypass grafting using a left internal mammary graft, an occluded right vertebral artery, and termination of the left vertebral artery in the posterior inferior cerebellar artery. In the absence of these absolute indications for revascularization, we manage patients expectantly and perform a subclavian-to-carotid transposition or carotid-subclavian bypass if signs of upper extremity ischemia or spinal ischemia develop.
Sizing and Device Selection
Choosing an appropriately sized endograft is critical in the treatment of a/sTBAD. There is evidence that the risk of RTAD increases with more aggressive oversizing.5-7 RTAD developed in 16 of the 1,010 patients (1.6%). For this reason, we size 1:1 (0%–5% oversizing) at the proximal (nondissected) aorta. Distally, we will usually tolerate a small degree of oversizing, as the consequences of stent graft–induced dissection in this location are less dire than those associated with RTAD. For the distal landing zone, we measure the entirety of the aortic diameter (TL + FL), as the septum in a/sTBAD is usually fairly pliable and should be expanded toward the outer wall of the aorta by the radial force of the endograft, increasing the likelihood of favorable remodeling.
In many cases, the aortic diameters are such that a single endograft (or two endografts with or without a tapered device) can be used to complete the repair and seal both proximally and distally. However, there are cases in which the discrepancy between the proximal and distal landing zones is pronounced with a significantly smaller distal aorta. During TEVAR for aneurysm, the smaller distal device can be implanted first with the larger proximal device landed within it. However, in the setting of a/sTBAD, placement of the distal piece may seal off a reentry tear, leading to increased pressure in the FL and increasing the risk for RTAD. In this setting, a three-piece repair can be implemented, with the proximal device being implanted first, then the distal smallest device, and last, a middle bridging device that is sized to seal within both the proximal and distal endografts.
In addition to sizing, the device structure appears to be a risk factor for complications including RTAD. A recent meta-analysis of 8,969 patients from 50 publications reported that the incidence of RTAD was higher in patients who had proximal bare stents, more proximal landing zones, and were treated for dissection compared to aneurysm.7
Length of Coverage
As previously stated, coverage of the primary entry tear is the principal goal in TEVAR for a/sTBAD. We consider an improvement in TL diameter and coverage of large distal fenestrations to be high-priority secondary goals. The desire to improve aortic remodeling with longer segment coverage has to be balanced with the risk of paraplegia due to intercostal artery coverage. We have noted that aortic remodeling often stops at the distal extent of the covered aorta. For this reason, we prefer to cover down to the level of the diaphragm to maximize the extent of aortic remodeling. In general, we use contrast angiography and intravascular ultrasound (IVUS) to aid us in determining the ideal amount of aortic coverage; if the mesenteric and renal vessels demonstrate brisk systolic filling and no signs of dynamic ostial compromise by the septum, we may leave the distal thoracic aorta uncovered.
INTRAOPERATIVE TECHNIQUES
We routinely employ several adjunctive techniques to facilitate efficient and effective TEVAR in the setting of a/sTBAD. We begin by using ultrasound guidance for femoral access. It is critical to visualize the common femoral bifurcation and the artery’s point of emergence from under the inguinal ligament to avoid low or high sticks. Preclosure is performed using the ABBOTT PERCLOSE® PROGLIDE Suture-Mediated Vessel Closure System, each rotated like a key approximately 45° off midline before firing to produce two crossing sutures for vessel closure.
We are proponents of IVUS in TEVAR for a/sTBAD for several reasons. During initial wire insertion, IVUS allows for confirmation of wire and catheter placement within the TL. It also helps to localize the primary entry tear and any significant distal fenestrations. Next, it can be used to rule out the presence of ascending aortic involvement and to confirm that the proximal landing zone is in an area of healthy aorta not affected by dissection. IVUS also allows confirmation of the aortic measurements obtained by CT scan, helping to reduce the chances of unintentional oversizing. Furthermore, IVUS can demonstrate the dynamic changes in TL and FL sizes throughout the cardiac cycle, both before and after device implantation. If we see an improvement in TL diameter after the proximal device implantation and no sizeable fenestrations between the trailing end of the endograft and the celiac axis, we may elect not to cover the remaining thoracic aorta. Finally, IVUS can assess branch vessel compromise and determine whether any additional treatment is indicated in cases of static obstruction.
In order to avoid RTAD and new distal dissection, we do not routinely perform balloon expansion of the endograft in cases of a/sTBAD. In fact, we will often tolerate a markedly compressed appearance of the graft, with the expectation that, over time, the outward radial force of the nitinol stent graft will gradually expand the TL and hopefully obliterate the FL.
After device implantation and confirmation of a satisfactory result with angiography and IVUS, we turn our attention to hemostasis in the groin(s). After cinching and locking the sliding knots of the ABBOTT PERCLOSE PROGLIDE Suture-Mediated Vessel Closure System, we feed them through a section of tubing cut from the side arm of a sheath to fashion a Rumel-type tourniquet, which holds additional pressure on the arteriotomy and provides a conduit to deliver a small aliquot of thrombin directly to the arterial wall to help achieve hemostasis. We have previously described this technique and find it very useful to prevent troublesome oozing or hematoma formation.8 With careful attention to meticulous technique during initial femoral access and this closure strategy, we are able to perform an entirely percutaneous operation in the vast majority of our TEVAR cases.
PERIOPERATIVE TECHNIQUES
Our perioperative management of a/sTBAD patients primarily revolves around preventing or treating spinal cord ischemia (SCI). We ask our anesthesiologist colleagues to place a lumbar drain (LD) preoperatively in almost all cases, unless: (1) the patient has hostile back anatomy (multiple lumbar operations or fusions, infection in the area, etc.); (2) the patient is hemodynamically unstable due to rupture; (3) the duration or severity of end-organ malperfusion suggests that rapid TL expansion is of paramount importance; or (4) the drain has been attempted several times by a senior attending anesthesiologist without success. If the drain cannot be placed, we generally follow these patients expectantly. However, a LD may need to be placed postoperatively as an attempt to rescue a case of postoperative SCI.
Once a LD is in place, management of cerebrospinal fluid (CSF) drainage proceeds according to our previously published protocols.9 In essence, this involves draining up to 15 mL/h of CSF in order to maintain CSF pressure < 10 mm Hg in a neurologically intact patient or draining an unlimited amount of CSF in order to maintain a CSF pressure < 5 mm Hg in a patient with SCI. Transfusion, supplemental oxygenation, and the administration of pressors or inotropes are also used as needed to optimize spinal cord perfusion pressure.
POSTOPERATIVE ISSUES
If no neurologic deficits are present, the LD is typically clamped on the morning of the first postoperative day and removed on the second postoperative day. Patients are usually seen in the clinic for follow-up with a postoperative CTA at 4 weeks. After that, they are seen at 6 and 12 months for a surveillance CTA and yearly thereafter (Figure 3).
CONCLUSION
Management of patients with a/sTBAD remains a challenge. Application of TEVAR as the treatment of choice for patients with complicated a/sTBAD reflects a significant paradigm shift in the last decade. There is an expanding role for TEVAR in patients with uncomplicated a/sTBAD with high-risk features, but additional studies are required to further define optimal patient selection and treatment strategies in this cohort. Our strategies, as previously detailed, have helped us to achieve favorable outcomes in the endovascular treatment of a/sTBAD.
- Ray HM, Durham CA, Ocazionez D, et al. Predictors of intervention and mortality in patients with uncomplicated acute type B aortic dissection. J Vasc Surg. 2016;64:1560-1568.
- Trimarchi S, Eagle KA, Nienaber CA, et al. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2010;122:1283-1289.
- VIRTUE Registry Investigators. The VIRTUE Registry of type B thoracic dissections—study design and early results. Eur J Vasc Endovasc Surg. 2011;41:159-166.
- VIRTUE Registry Investigators. Mid-term outcomes and aortic remodelling after thoracic endovascular repair for acute, subacute, and chronic aortic dissection: the VIRTUE Registry. Eur J Vasc Endovasc Surg. 2014;48:363-371.
- Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg. 2014;260:389-395.
- Liu L, Zhang S, Lu Q, et al. Impact of oversizing on the risk of retrograde dissection after TEVAR for acute and chronic type B dissection. J Endovasc Ther. 2016;23:620-625.
- Chen Y, Zhang S, Liu L, et al. Retrograde type A aortic dissection after thoracic endovascular aortic repair: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e004649.
- Furlough CL, Desai SS, Azizzadeh A. Adjunctive technique for the use of ProGlide vascular closure device to improve hemostasis. J Vasc Surg. 2014;60:1693-1694.
- Estrera AL, Sheinbaum R, Miller CC, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg. 2009;88:9-15; discussion 15.
Products listed may not be available in all markets.
See all products by region:
Bruce Tjaden, MD
Department of Cardiothoracic and Vascular Surgery
The University of Texas Health Science Center
Houston, Texas
Disclosures: None.
Ali Azizzadeh, MD, FACS
Professor and Director of Vascular Surgery
Vice Chair, Department of Surgery
Associate Director, Heart Institute
Cedars-Sinai Medical Center
Los Angeles, California
Disclosures: Consultant to Gore & Associates and Medtronic.
