Advancing endovascular options: New endovascular devices and insights to extend aortic treatment to more patients
ADVANCING ENDOVASCULAR OPTIONS
Sponsored by Gore & Associates
Early results and lessons learned with the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
Device-related factors that are designed to overcome the existing limitations in TEVAR.
Dittmar Böckler, MD
Moritz S. Bischoff, MD
Vera Zipse, BSc
Philipp Geisbüsch, MD
Over the past 3 decades, since its introduction by Dake et al in the 1990s,1 thoracic endovascular aortic repair (TEVAR) has become the first-line modality for the treatment of thoracic aortic pathologies.2 Clinical registries such as the RESTORE II registry,3 the TRAVIATA registry,4 and the multicenter European Conformable GORE® TAG® Thoracic Endoprosthesis Registry5 demonstrated that a majority of aortic pathologies involve the arch with landing zones 0–3.6 Many parameters influence technical and clinical outcomes in TEVAR due to the arch (Figure 1). Besides patient-related (eg, underlying disease or emergency setting) and procedure-related factors (eg, intraoperative imaging), device-related parameters such as stent graft design, conformability, radial force, and placement accuracy significantly contribute to the early technical success of the procedure.
TEVAR IN THE AORTIC ARCH
The aortic arch is a challenging environment for many anatomical and device attribute reasons such as tortuosity, the windsock effect, and others. As the indications for TEVAR expanded over the years to include patients with unfavorable anatomy, the limitations of some stent grafts became evident. Many patients have tortuous, calcified, or even small access vessels. Additionally, tortuosity of the descending thoracic aorta and angulation of the aortic arch makes trackability of most delivery systems difficult, increasing the risk of peripheral and cerebral embolization or vessel wall damage. Rupture of iliac vessels and retrograde dissection of the aortic arch have been described as possible complications of thoracic stent grafting.7,8 The “gothic” arch configuration and the high-pressure environment in the aortic arch may lead to failure in trackability/delivery and deployment issues, thus contributing to type I endoleaks. The design of most thoracic stent grafts was derived from experience with devices for the infrarenal aorta. Furthermore, the currently available devices were intended to treat the straight tube segment of the descending aorta, and so deployment of a stent graft in the arch remains challenging.
CURRENT LIMITATIONS OF PERFORMING TEVAR
In general, current device-related limitations while performing TEVAR are introduction profile (up to 24 F), accuracy and control during deployment, stent graft conformability, inner curve apposition, preservation of aortic branches, and long-term durability. Additionally, other limitations such as stroke, spinal cord injury, and endoleaks are well documented in the literature.
Use of a device in the distal aortic arch requires perfect conformability and good apposition to the inner curve of the aortic wall to avoid endoleaks, migration, or compression/collapse. Severe arch angulation resulting in lack of inner curve apposition remains a well-documented limitation of commercially available thoracic devices. Therefore, further product research and device development were necessary to produce devices specifically designed to meet the requirements of patients with different pathologies and treatment in different segments of the aortic arch and descending thoracic aorta.
To better understand and address these clinical challenges and design issues, Gore & Associates created benchtop models to replicate real patient anatomies with severe arch angulation and challenging landing zones. This research supported the development of the next-generation device, which fulfills the need for a conformable thoracic stent graft to meet a broad range of anatomies, combined with an innovative deployment system that provides enhanced control, positioning, and wall apposition during deployment: the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System.*
STENT GRAFT DEVELOPMENT AND EVOLUTION OF THE NEXT-GENERATION SYSTEM
Since its first commercial use in 1998, more than 125,000 GORE® TAG® Devices have been distributed worldwide. The original GORE TAG Device was modified in 2003 to eliminate the clinically observed spine wire fracture failure mode. Innovation of stent graft design and improvement of device performance was continued with the Conformable GORE TAG Thoracic Endoprosthesis, which was engineered for endovascular repair of the descending thoracic aorta with special consideration given to the unique anatomic and physiologic characteristics of patients presenting with pathologies involving the aortic arch such as aneurysm, traumatic aortic transection, and aortic type B dissection. The new GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System, which recently became commercially available in Europe, maintains all attributes of the Conformable GORE TAG Device and adds new tools (Figure 2).
The new delivery system enhances control during deployment and vessel wall apposition. Compared to the current one-step deployment that opens the stent graft from the center toward both ends, the new staged deployment first opens the stent graft to an intermediate diameter from leading to trailing end. This is then followed by a separate handle action that completes stent graft expansion to full diameter from trailing to leading end.
Angulation along the inner curvature is possible at both intermediate- and full-diameter stages. It is optional and can be skipped at the discretion of the implanting physician. It is important to remember that angulation is irreversible; however, the auditory, visual, and tactile feedback during angulation allows for a slow and controlled adjustment of orthogonal positioning of the proximal end of the stent graft (Figure 3).
The olive is now precurved and is designed to facilitate self-orientation of the stent graft to the outer curve in the aortic arch. A lock wire keeps the stent graft attached to the catheter throughout the procedure. In comparison to the current Conformable GORE TAG Device, the new-generation device comes with two constraining sleeves rather than one.
The two case reports that follow demonstrate our early experience with the new GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System.
CASE REPORT 1
A 40-year-old man presented with an asymptomatic progressive postcoarctation aneurysm of 42 mm diameter after two previous open surgical repairs. He was treated with TEVAR in July 2017 at our institution to prevent rupture and aortic-related death. The left subclavian artery (LSA) was revascularized by a staged left carotid-subclavian bypass to create a sufficient proximal landing zone and to prevent endoleak from the LSA. After percutaneous right common femoral access was achieved, routine implantation steps for the GORE TAG Conformable Thoracic Stent Graft were followed using a double-curved stiff wire nested into the aortic valve, eliminating stored energy by first advancing past the target location and pulling the device back to the intended landing zone and by pushing the guidewire and device to the outer curvature during deployment. Upon deployment to intermediate diameter, the gold band demarking the edge of the graft material was clearly observed. At this intermediate-diameter stage, parallax correction was used to optimize device positioning before initiating full and final deployment (Figure 4). Pre- and postoperative images demonstrating successful exclusion of the aneurysm are shown in Figure 5.
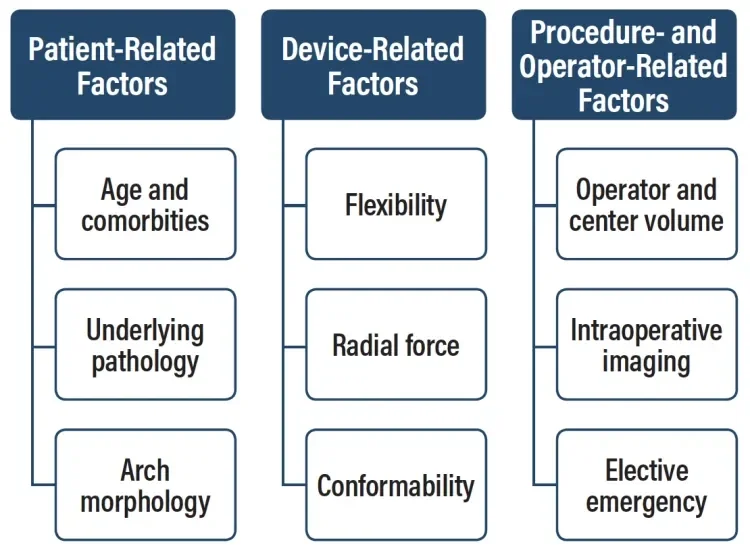
Figure 1. Factors influencing technical and clinical outcomes in TEVAR. Adapted from Böckler D. TEVAR in the aortic arch—influence of the proximal landing zone on outcome. In: Greenhalgh RM, ed. Vascular and Endovascular Controversies Update: 1978–2015. London, UK: BIBA Medidcal; 2015.
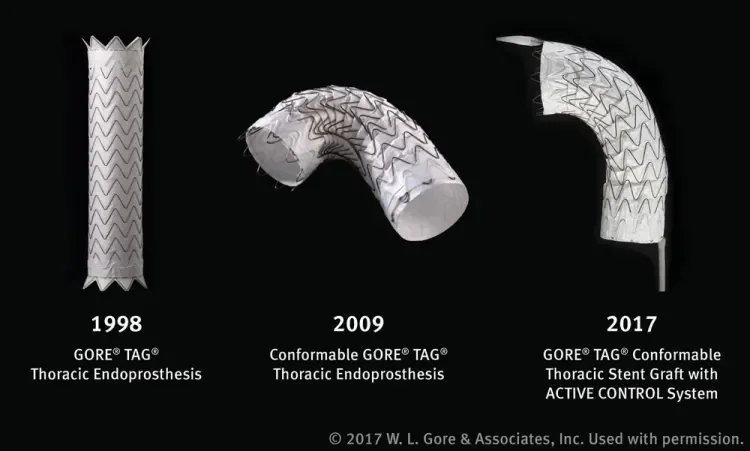
Figure 2. The evolution of Gore’s TEVAR device, from the original GORE® TAG® Device to the Conformable GORE® TAG® Endoprosthesis and, most recently, the new GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System.
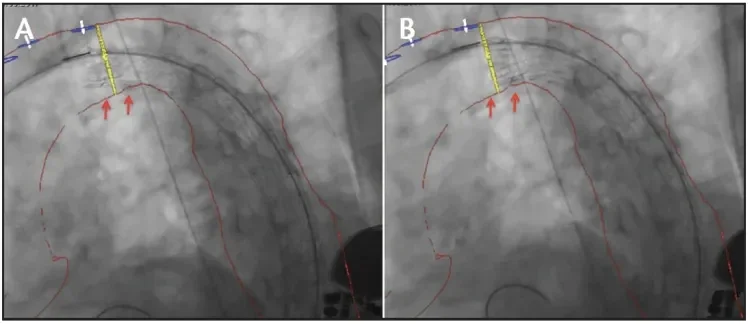
Figure 3. Intraoperative fluoroscopy before (A) and after (B) angulation and refinement of stent graft position at the intermediate stage of deployment. The red arrows indicate the improved apposition of the device at the inner arch curvature.
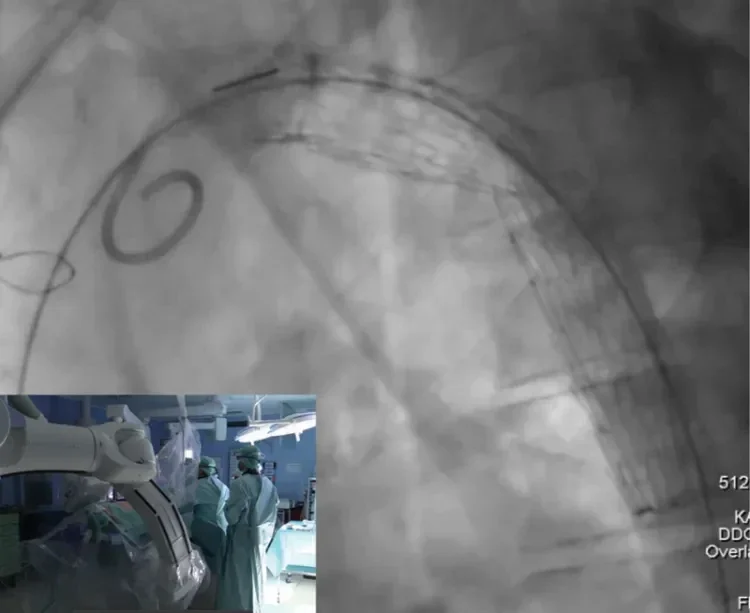
Figure 4. On intraoperative fluoroscopy, parallax correction of the C-arm was adjusted according to the gold band marker on the proximal end of the device. A perpendicular angle to the aorta can be confirmed when the gold band appears as a straight line, rather than an ellipse.
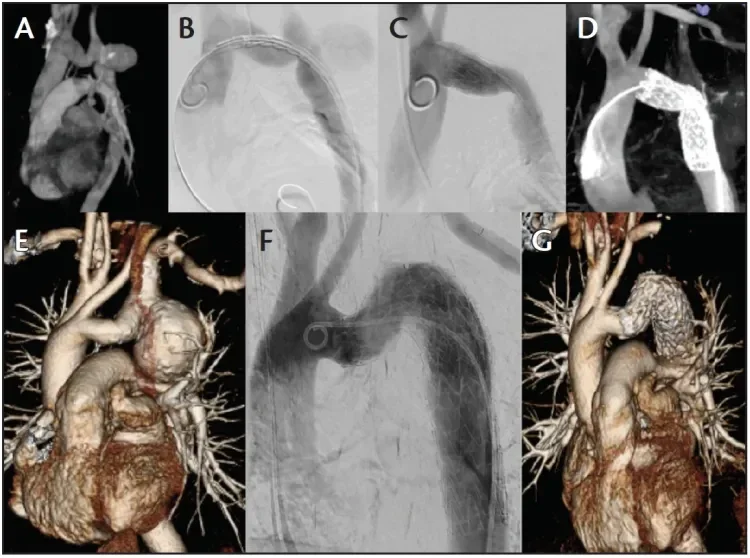
Figure 5. Preoperative three-dimensional (3D) CTA (A), intraoperative digital subtraction angiography at predeployment (B) and after full deployment (C), and intraoperative on-table cone-beam CTA (D) of a successful GORE® TAG® Conformable Thoracic Stent Graft implantation in a 40-year-old man with a postcoarctation aneurysm of 42 mm. The bottom three images demonstrate preoperative
3D CT reconstruction (E), completion angiography (F), and postoperative 3D CT reconstruction (G).
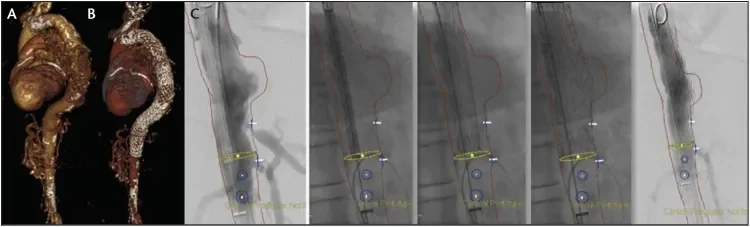
Figure 6. Pre- and postoperative CT scans shows the thoracic aneurysm of the patient before (A) and after (B) treatment. Intraoperative placement of the distal device at the level of the celiac trunk (C).

Figure 7. The device is constrained within two sleeves, which stay in place and are “sandwiched” between the stent graft and the aortic wall at the outer curve after deployment. The precurved olive is designed to enable self-orientation of the device toward the outer aortic curve.

CASE REPORT 2
A 72-year-old woman with a thoracic descending aortic aneurysm (62 mm maximum diameter) presented for endovascular treatment. The distal landing zone was 20 mm in length. Using fusion imaging and apnea, the third, most distal device was placed just at the level of the celiac trunk. The deployment was precise and accurate. This was enabled by the secondary deployment to full diameter opening from trailing to leading ends, which is a unique feature in the design of TEVAR devices (Figure 6).
DISCUSSION
With respect to our site’s early experience of 15 cases with the GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System for different indications, we have learned that staged deployment enables refinement of device placement and angulation, especially in the intermediate phase. Because the device is designed to ensure continuous blood flow throughout the procedure, it enables precise stent graft placement without any time pressure, which is a new and beneficial experience for operators. The hemodynamic stability led our site to abandon rapid pacing altogether when using this device. Staged deployment also allows for visualization of stent graft positioning and parallax correction according to the radiopaque gold band at the proximal stent graft end and to refine device placement with additional angiography, if needed. Optional angulation was used in 40% of all procedures so far. Although the partially uncovered stent row has always been part of the landing zone per the Conformable GORE TAG Device’s instructions for use, the presence of two sleeves instead of one makes it even more critical to case plan accordingly (Figure 7).
The secondary deployment sequence to full diameter from trailing to leading ends, combined with the attachment of the device to the catheter via the lock wire, provides full control during the entire deployment process. The device was preferably chosen for patients with short or challenging distal landing zones close to the celiac trunk or superior mesenteric artery. For this reason, the GORE TAG Conformable Thoracic Stent with ACTIVE CONTROL System is now our first-choice stent graft, not only in the arch (zone 0–2, especially in dissection and trauma cases) but also in zone 4.
The profile of all available sizes (21–45 mm) has not changed compared to the Conformable GORE TAG Device, but access problems have not been an issue in our experience so far. This new device is conceptually designed to overcome the previously described limitations of TEVAR devices that are currently commercially available on the market.
The Benefits of the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System sidebar summarizes my personal arguments of why this became my device of first choice since its commercial launch in Europe in July 2017.
CONCLUSION
Based on our preliminary experience, the staged deployment and optional angulation of the new GORE TAG Conformable Thoracic Stent Graft optimizes device placement and accuracy. Early experience is extremely convincing and preliminary results are very promising. The device is designed to overcome the described existing limitations in TEVAR. Larger studies and longer follow-up are needed to prove these early observations. The SURPASS registry has already begun in Europe to address these endpoints.
- Dake MD, Miller DC, Mitchell RS, et al. The “first generation” of endovascular stent-grafts for patients with aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg. 1998;116: 689-703; discussion 704.
- Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85(1 suppl):S1-41.
- Riambau V, Zipfel B, Coppi G, et al; RELAY Endovascular Registry for Thoracic Disease RESTORE Investigators. Final operative and midterm results of the European experience in the RELAY Endovascular Registry for Thoracic Disease(RESTORE) study. J Vasc Surg. 2011;53:565-573.
- Torsello GB, Torsello GF, Osada N, et al. Midterm results from the TRAVIATA registry: treatment of thoracic aortic disease with the valiant stent graft. J Endovasc Ther. 2010;17:137-150.
- Böckler D, Brunkwall J, Taylor PR, et al. Thoracic endovascular aortic repair of aortic arch pathologies with the conformable thoracic aortic graft: early and 2 year results from a European Multicenter registry. Eur J Vasc Endovasc Surg. 2016;51:791-800.
- Mitchell RS, Ishimaru S, Criado FJ, et al. Third International Summit on Thoracic Aortic Endografting: lessons from long-term results of thoracic stent-graft repairs. J Endovasc Ther. 2005;12:89-97.
- Leurs LJ, Bell R, Degrieck Y, et al; Eurostar and the UK Thoracic Endograft Registry collaborators. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg. 2004;40:670-679; discussion 679-680.
- Canaud L, Ozdemir BA, Patterson BO, et al. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg. 2014;260:389-395.
Products listed may not be available in all markets.
See all products by region:
Dittmar Böckler, MD
Department of Vascular and Endovascular Surgery
Heidelberg, Germany
Disclosures: Receives speaker honoraria and consultant fees from Gore & Associates.
Moritz S. Bischoff, MD
Department of Vascular and Endovascular Surgery
Heidelberg, Germany
Disclosures: None.
Vera Zipse, BSc
Department of Vascular and Endovascular Surgery
Heidelberg, Germany
Disclosures: None.
Philipp Geisbüsch, MD
Department of Vascular and Endovascular Surgery
Heidelberg, Germany
Disclosures: None.
