Pre-Clinical and Clinical Data
Outcomes: Hernia Recurrence Rates
889 patients in clinical literature, including a multicenter prospective clinical study, provide data to show GORE® BIO-A® Tissue Reinforcement is an excellent choice in soft tissue repair.* GORE® BIO-A® Tissue Reinforcement has low recurrence rates when used in hiatal hernia repair.1,2,3
Pre-Clinical Data
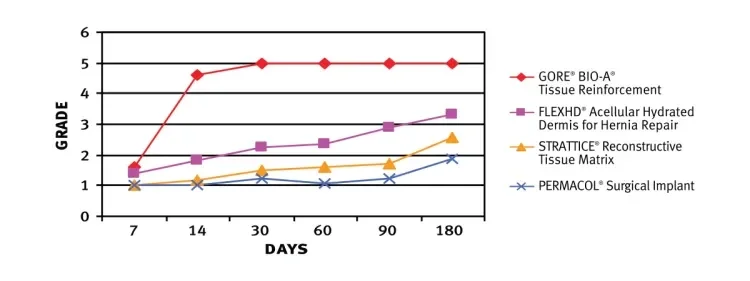
GORE® BIO-A® Tissue Reinforcement exhibited higher degree of cellular and vascular in-growth and collagen deposition than three commonly used biologic meshes in a sterile field. The use of a polyglycolic acid/trimethylene carbonate absorbable mesh results in a favorable tissue response.4
Paraesophageal/Hiatal Hernia Repair
| Authors & Journal | Facts | Highlighted Data |
|---|---|---|
|
Silecchia G, Iossa A, Cavallaro G, Rizzello M, Longo F. Minimally Invasive Therapy & Allied Technologies 2014;23(5):302-308. |
|
43 patients 17.4 months follow up |
| Authors & Journal | Facts | Highlighted Data |
|---|---|---|
|
Alicuben ET, Worrell SG, DeMeester SR. American Surgeon 2014;80(10):1030-1033. |
|
114 patients 12 months follow up |
| Authors & Journal | Facts | Highlighted Data |
|---|---|---|
|
Gebhart A, Vu S, Armstrong C, Smith BR, Nguyen NT. American Surgeon 2013;79(10):1017-1021. |
|
92 patients 30 months follow up |
Complex Ventral Hernia
| Authors & Journal | Facts | Highlighted Data |
|---|---|---|
|
Rosen M, Bauer J, Carbonell A, Cobb W, Matthews B, Selzer D, |
|
104 patients 15 months follow up |
| Authors & Journal | Facts | Highlighted Data |
|---|---|---|
|
Jacobsen G, Chao J, Sandler B, Barajas-Gamboa J, Macias C, Talamini M. Hernia |
|
93 patients 9 months follow up |
Ostomy Reversal
| Authors & Journal | Facts | Highlighted Data |
|---|---|---|
|
Pandey SR, Najafian H, Ramanujam K, Ramanujam P. Hernia |
|
50 patients 24 months follow up |
References
- Birk D, von Heesen M. Tissue reinforcement with Gore BioA in large hiatal hernias. A prospective clinical study. Abstract presented at the 15th Annual Hernia Repair Meeting; March 13-16, 2013; Orlando, FL. Hernia 2013;17(Supplement 1):S85.
- Massullo JM, Singh TP, Dunnican WJ, Binetti BR. Preliminary study of hiatal hernia repair using polyglycolic acid : trimethylene carbonate mesh. Journal of the Society of Laparoendoscopic Surgeons 2012;16(1):55-59.
- Jethwa P, Sriskandarajah K, James A. Laparoscopic hiatus hernia repair with Gore Bio-A mesh: a pilot study. Abstract presented at the 33rd International Congress of the European Hernia Society; May 10-13, 2011; Ghent, Belgium. Hernia 2011;15(Supplement 2):S60. Abstract P-076.
- Zemlyak AY, Colavita PD, Tsirline VB, et al. Absorbable glycolic acid/trimethylene carbonate synthetic mesh demonstrates superior in-growth and collagen deposition. Presented at: 2012 Abdominal Wall Reconstruction Conference; June 13-16, 2012; Washington, D.C.
*Data on File
STRATTICE® is a trademark of LifeCell. MAXON®, PERMACOL are trademarks of Covidien AG or its affiliates. FLEXHD® is a trademark of Ethicon, Inc. ALLOMAX, XENMATRIX®, COLLAMEND®, are trademarks of C. R. Bard, Inc. SURGIMEND® is a trademark of TEI Biosciences, Inc. SURGISIS® is a trademark of Cook Medical, Inc. VERITAS® is a trademark of Synovis Surgical Innovations, Inc.