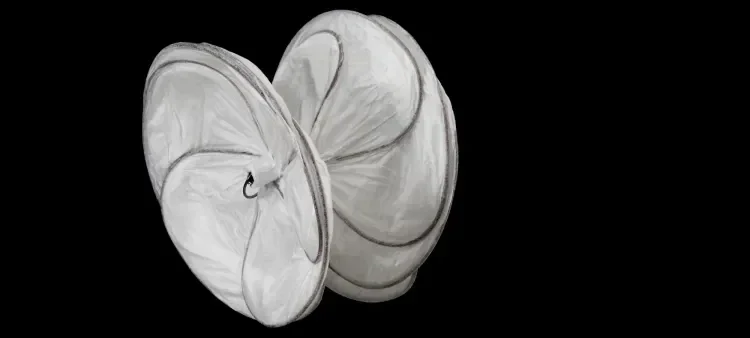
GORE® CARDIOFORM Septal Occluder
Designed to perform with the natural anatomy of the heart, the GORE® CARDIOFORM Septal Occluder is a soft and conformable device for transcatheter closure of ASDs and PFOs up to 17 mm, including challenging defects.
Progressive approach to PFO closure
As a result of Gore’s long-standing effort to solve complex challenges through physician collaboration, this innovative minimal wall injury design features two independent, conformable discs to span and cover the anatomy, allowing for rapid endothelialization. It is a permanent implant consisting of a nitinol wire frame covered with a thin ePTFE membrane. The ePTFE material, invented and manufactured by Gore, has been used in open-heart surgery for more than 40 years with a history of proven safety in medical implants.
PFO closure with the GORE® CARDIOFORM Septal Occluder offers an advanced solution for stroke teams and their cryptogenic stroke patients. This approach to stroke prevention is proven in the Gore REDUCE Clinical Study, the only U.S. IDE trial that achieved its primary end point and showed the largest reduction in recurrent ischemic stroke*,1 in all PFO shunt sizes over medical therapy alone.
REDUCE Clinical Study
Clear data. Confident stroke prevention.
- Groundbreaking 77% relative reduction in recurrent stroke for cryptogenic stroke patients*,1
- Primary analysis published in The New England Journal of Medicine
- Rapid demonstration of statistically significant stroke reduction: Met primary endpoint in the intention-to-treat analysis*,1 with an average follow-up of 3.4 years
- Conforms for confident closure with rapid tissue ingrowth: 98% effective closure rate†,1
- Low device/procedure SAE rates: Device-related SAE 1.4%, procedure-related SAE 2.5%
- Robust trial design: Standardized antiplatelet therapy for the medical arm with prospective, 2-year MRI imaging
- Inclusive of all PFO types: The only trial to reach primary end point and include all PFO anatomies and shunt sizes*,1
* The REDUCE study determined safety and efficacy of patent foramen ovale (PFO) closure with the GORE® CARDIOFORM Septal Occluder or GORE® HELEX® Septal Occluder plus antiplatelet medical management compared to antiplatelet medical management alone in patients with a PFO and history of cryptogenic stroke. All PFO anatomies were incorporated into this study within indicated sizing parameters of the IFU.
† GORE® CARDIOFORM Septal Occluder effective closure rate results in device group subjects who received a study device. Effective closure defined as freedom from large shunt > 25 bubbles as detected by transthoracic echocardiography adjudicated by echo core lab.
1.) Søndergaard L, Kasner SE, Rhodes JF, et al. Gore REDUCE Study Investigators. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. New England Journal of Medicine 2017;377(11):1033-1042.
Featured resources
