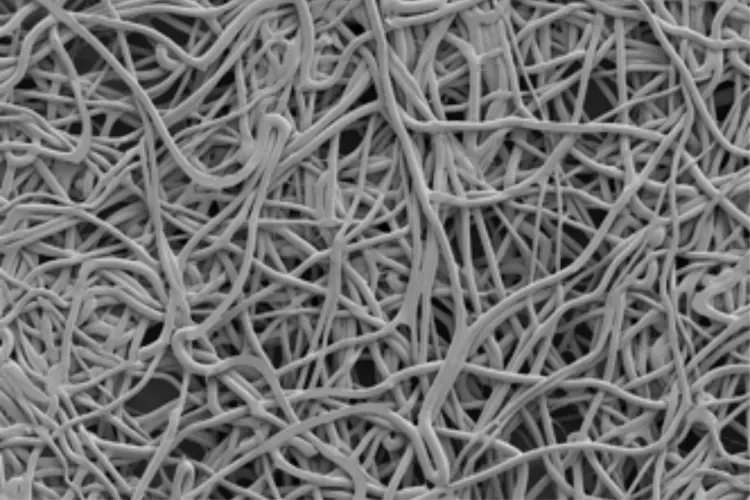
Proven Material
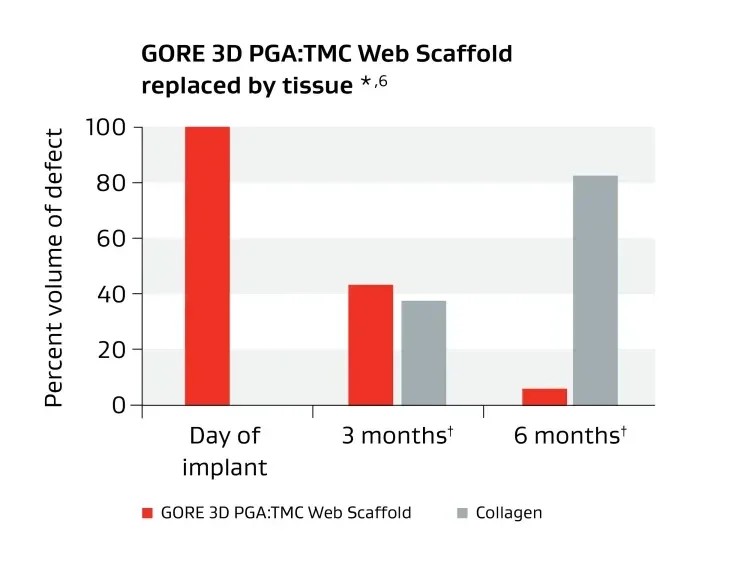
Unique biosynthetic scaffold designed to facilitate tissue generation.1
Strength and flexibility
The only staple line reinforcement material with trimethylene carbonate (TMC) to help maintain the strength with increased conformability.2
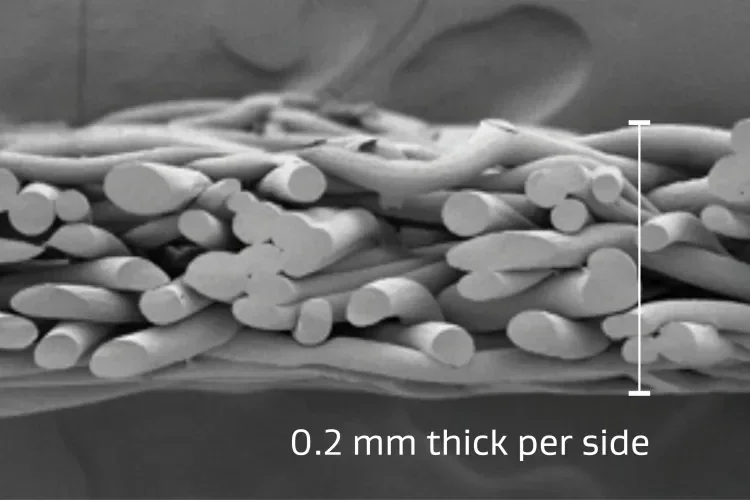
Material thickness
Thin, conformable and compressible.
Consistent 0.4 mm average total thickness allowing for even pressure distribution over the tissue while minimizing impact on staple formation. The composition of GORE® SEAMGUARD® Reinforcement allows for the thickness to compress when more force is applied.3
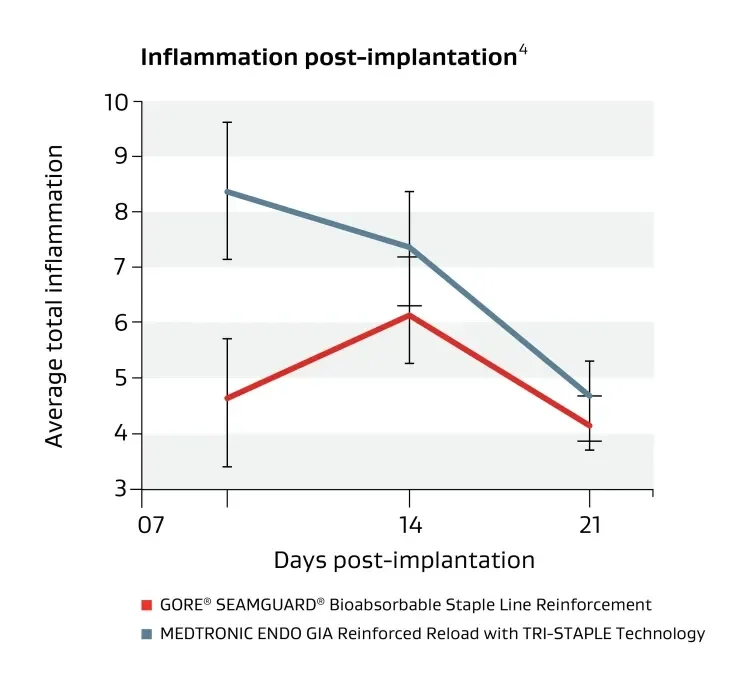
Holds and protects
Strengthens staple lines with minimal inflammation.
Made with a synthetic buttressing material engineered to reduce perioperative leaks and bleeding.7 Compared with similar materials it has shown minimal tissue inflammatory response.2,4-5

* Data on file 2015; W. L. Gore & Associates, Inc; Flagstaff, AZ.
† Cells and blood vessels make up remaining volume.
- Zemlyak AY, Colavita PD, Tsirline VB, et al. Absorbable glycolic acid/trimethylene carbonate synthetic mesh demonstrates superior in-growth and collagen deposition. Presented at the Abdominal Wall Reconstruction (AWR) Meeting; June 14-16, 2012; Washington, DC. Abstract 35.
- Katz AR, Mukherjee DP, Kaganov AL, Gordon S. A new synthetic monofilament absorbable suture made from polytrimethylene carbonate. Surgery, Gynecology & Obstetrics 1985;161(3):213-222.
- Pertuit A. Bioabsorbable Seamguard Density study for Leak Testing. Flagstaff, AZ: W L. Gore & Associates. Inc; 2003. [Work plan] MD15039.
- Khaitan L, Yoo J. Comparison Between Staple Line Reinforcement Materials: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement and MEDTRONIC ENDO GIA Reinforced Reload with TRI-STAPLE Technology. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2017. [Case series]. AV1650-EN1.
- Crawford N. Assessment of Various Staple Line Reinforcement materials in a Porcine Model. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2015. [Histopathology report]. Experiment 2284TL. T3 Protocol WG03P.
- Morales-Conde S, Flores M, Fernández V, Morales-Méndez S. Bioabsorbable vs polypropylene plug for the “Mesh and Plug” inguinal hernia repair. Poster presented at the 9th Annual Meeting of the American Hernia Society; February 9-12, 2005; San Diego, CA. AJ0049-EN2.
- McCrea C. GORE® SEAMGUARD® Reinforcement Product Family: Relationship between Design Inputs and “Engineered to reduce the incidence of perioperative leaks and bleeding” Marketing Statement. Flagstaff, AZ; 2015. [Work plan]. WP107241.
MEDTRONIC, ENDO GIA and TRI-STAPLE are trademarks of Medtronic, Inc.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231325377-EN