GORE® Tri-Lobe Balloon Catheter
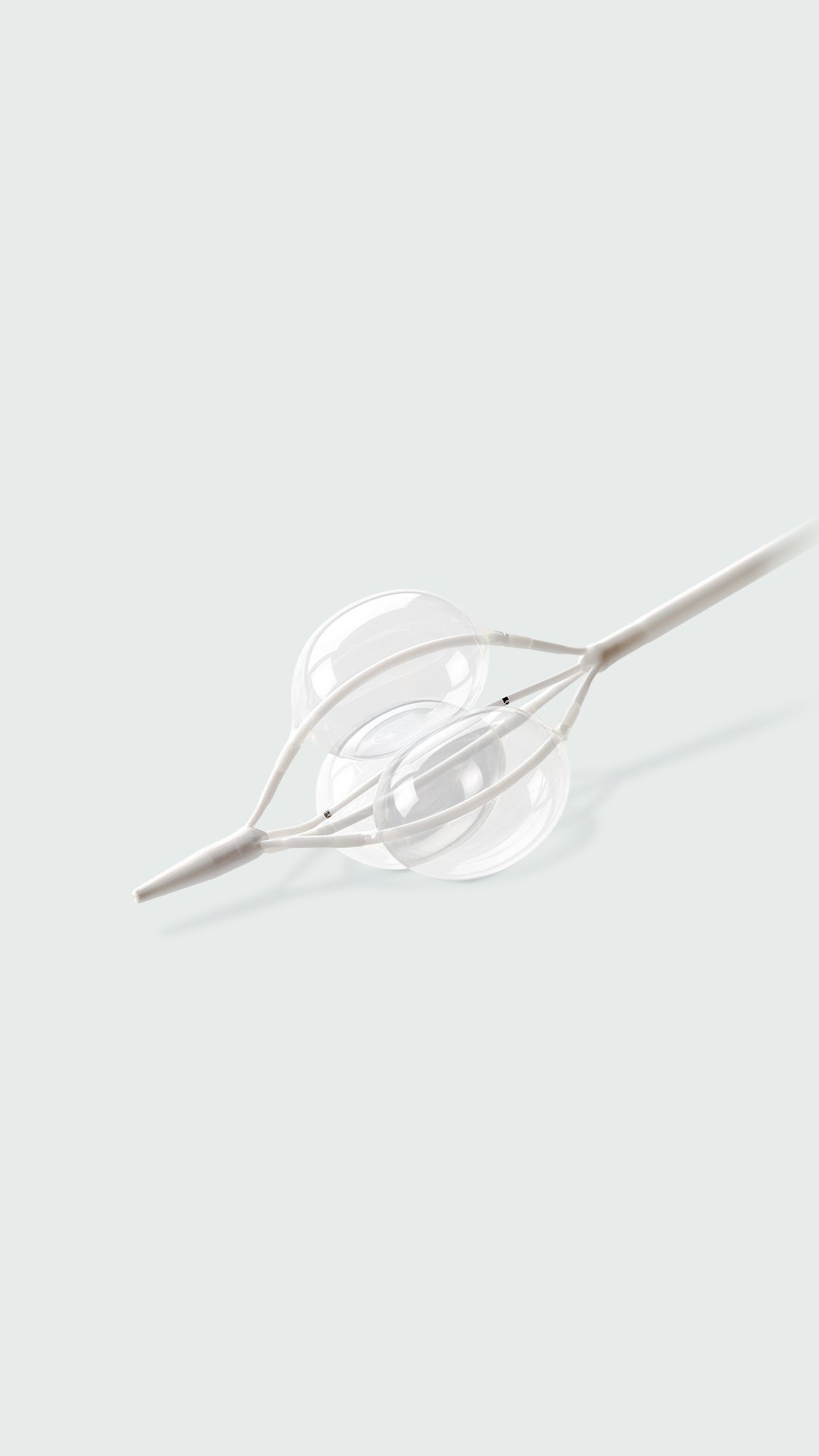
Continuous blood flow. Minimized potential for endoprosthesis movement.
The GORE® Tri-Lobe Balloon Catheter provides surgeons with greater stability and control in positioning endovascular grafts.
The GORE® Tri-Lobe Balloon Catheter is engineered to facilitate endovascular procedures through several key features:
- Device stability: Reduced endoprosthesis movement during positioning.
- Continuous blood flow: Our unique1,2,3 tri-lobe design allows continuous blood flow around the lobes while inflated.
- Compliant construction: Compliant polyurethane balloons offer exceptional conformance to the endoprosthesis.
- Greater control: Rapid, uniform and simultaneous balloon lobe inflation/deflation minimizes potential blood pressure spikes by not occluding the aorta when balloons are fully inflated.
The GORE® Tri-Lobe Balloon Catheter provides surgeons with higher degree of control in positioning endovascular grafts. Our innovative device is available in two sizes compatible with 18 Fr introducer sheaths. The small size is designed for 16–32 mm vessel diameters (for use with the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System, the GORE® TAG® Thoracic Branch Endoprosthesis or the GORE® EXCLUDER® AAA Endoprosthesis). The large size is designed for 26–42 mm vessel diameters (for use with the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System or the GORE® TAG® Thoracic Branch Endoprosthesis).
1. Bloss R, Krall B. Balloon Testing on Pulse Duplicator. Flagstaff, AZ: W.L. Gore & Associates, Inc.: 2009.
2. 2nd reference: Gendron M. Simulated Use Testing of Aortic Balloon Catheter for Design Verification - Final Amendment. Flagstaff, AZ: W.L. Gore & Associates, Inc; 2008. [Work plan]. MD39672.
3. [Technology notebook]. 1088.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® Tri-Lobe Balloon Catheter is indicated to facilitate in the endovascular repair of the thoracic or abdominal aorta due to lesions including aneurysms, dissections, trauma, and penetrating aortic ulcers.
CONTRAINDICATIONS: There are no known contraindications.