Clinical Outcomes
Long-term results you can trust
U.S. Pivotal Clinical Trials: Five year data across ALL etiologies.
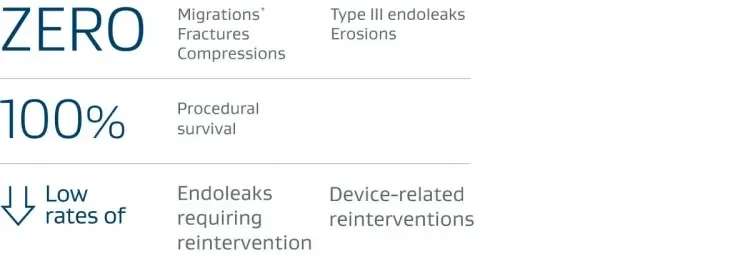
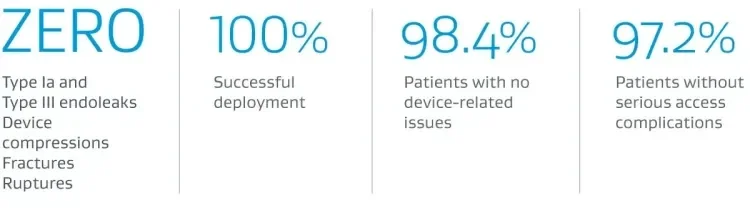

5-year Outcomes: U.S. IDE Clinical Trial, Traumatic Aortic Transection
Mark Farber, M.D., National Principal Investigator provides his perspective on key study outcomes
Time-tested innovation

* Migrations are reported as those requiring reintervention.
† Rates are based on physician experience as reported for 127 subjects in Europe within a 30 day follow-up period. European-Post Approval Registry: Observational Registry Characterizing the Performance and Feature Use of the GORE® TAG® Conformable Thoracic Stent Graft Featuring ACTIVE CONTROL System. (data on file 2017; W.L. Gore & Associates, Inc; Flagstaff, AZ.)
‡ Note: Only serious endoleaks are reported in GREAT (i.e., requiring reintervention).
§ Data on file; W.L. Gore & Associates, Inc.; Flagstaff, AZ.
- W. L. Gore & Associates. 'GREAT' Global Registry for Endovascular Aortic Treatment - Outcomes Evaluation. Bethesda, MD: National Library of Medicine; 2012. Available from: NLM Identifier: NCT01658787. Published August 7, 2012. Updated: September 29, 2021. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT01658787

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for markets where this product is available. RXOnly
Related to this product
231157293-EN
