Lesions across the elbow
Endovascular management of lesions at points of flexion that require stenting is challenging
- Stent grafts must be able to conform to the anatomy to provide thorough range of motion and be durable to resist fractures
- Flexion points in the circuit outside of the elbow are often underappreciated
- Positional studies highlight points of flexion outside of the elbow1
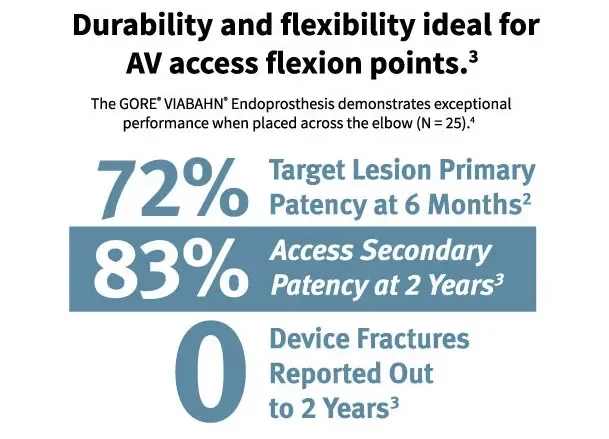
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*,† is engineered for long-term performance in areas of flexion
Proven Patency
- When placed across the elbow:
- 72% target lesion six-month primary patency2
- 67% circuit six-month primary patency2
Demonstrated durability
- 83% access secondary patency and zero device fractures at two years when placed across the elbow3
- Across all uses, the GORE® VIABAHN® Endoprosthesis has a reported fracture rate of < .015% (data on file 2018; W. L. Gore & Associates, Inc; Flagstaff, AZ.)
Conformable yet durable design
- Like with all Gore single nitinol wire stents, the design and frame construction reduces strain to provide mechanical durability
- Proven flexibility maintains flow at points of flexion1
The [GORE® VIABAHN® Endoprosthesis] has the flexibility, durability, and the indication to successfully treat lesions across the antecubital fossa. In my experience, the device does not compress or kink when the extremity is flexed as compared with other stent grafts…
-William DaVanzo, M.D.
Connect with a Gore Field Sales Associate
* As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in some regions.
- Webb M. Flow disturbances of upper arm graft outflow uncovered by positional studies. Endovascular Today 2014;13(6)Supplement:31-33.
- Vesely T, DaVanzo W, Behrend T, Dwyer A, Aruny J. Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. Journal of Vascular Surgery 2016;64(5):1400-1410.e1. http://www.sciencedirect.com/science/article/pii/S0741521416301756
- W. L. Gore & Associates, Inc. GORE® VIABAHN® Endoprosthesis versus Percutaneous Transluminal Angioplasty (PTA) to Revise Arteriovenous Grafts at the Venous Anastomosis in Hemodialysis Patients. (GORE REVISE Study, AVR 06-01). Flagstaff, AZ: W. L. Gore & Associates, Inc; 2012. [IDE Final Clinical Study Report]. G070069.
- GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD174703.