Long SFA lesions
Consider the demands of treating long-length lesions of the superficial femoral artery (SFA)
- Lesion length is a predictor of patency outcomes for several treatment modalities
- Drug-eluting stents
- Lesion length has been shown to be an independent predictor of restenosis1
- In a randomized study, COOK® ZILVER® PTX® Drug-Eluting Peripheral Stent has been shown to have lower patency in long lesions > 10 cm compared to those 10 cm or shorter2
- Drug coated balloon (DCB)
- Multivariate analysis of the Medtronic IN.PACT Global Study demonstrated that increasing lesion length was a predictor of increased risk for Clinically Driven Target Lesion Revascularization (CD TLR)3
- Bare-metal stent (BMS)
- The VIASTAR Trial demonstrated BMS had lower patency when treating lesions > 20 cm than when treating lesions shorter than 20 cm4
- Drug-eluting stents
- Complications increase with lesion length
- Risk of dissection when using DCB increases with lesion length5
- For many BMS, the risk of in-stent restenosis and stent fracture increases with lesion length6-9
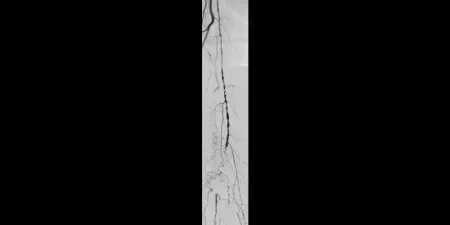
Before
Proximal SFA disease and mid-SFA occlusion
Images courtesy of James Persky, M.D. Used with permission.
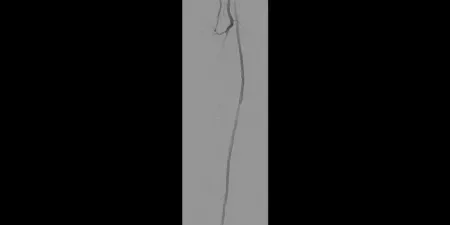
After
Post-placement of three 5 mm GORE® VIABAHN® Devices
Images courtesy of James Persky, M.D. Used with permission.
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*,† has demonstrated excellent patency and durability independent of lesion length4, 10-15
Proven patency
- 88% primary patency at one year in lesions 22 cm in length12
- 80% average primary patency demonstrated across seven multicenter, prospective, randomized or single arm studies with an average lesion length of 23 cm4, 10-15
Demonstrated durability
- 97% three-year secondary patency in complex disease (27 cm average lesion length, 93% chronic total occlusions)16
- Comparable clinical results to above knee surgical bypass (both prosthetic17 and native vein11)
Connect with a Gore Field Sales Associate
* As used by Gore, PROPATEN Bioactive Surface refers to Gore's proprietary CBAS Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in some regions.
- Iida O, Takahara M, Soga Y, et al; ZEPHYR Investigators. 1-year results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): predictors of restenosis. JACC: Cardiovascular Interventions 2015;8(8):1105-1112.
- Bausback Y. 2 year results of the REAL PTX Randomized Clinical Trial comparing Zilver PTX DES vs. DCB in femoropopliteal lesions. Presented at the 7th Munich Vascular Conference (MAC); December 7-9, 2017; Munich, Germany.
- Micari A, Brodmann M, Keirse K, et al; IN.PACT Global Study Investigators. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2-year results from the IN.PACT Global Study. JACC: Cardiovascular Interventions 2018;11(10):945-953.
- Lammer J, Zeller T, Hausegger KA, et al. Sustained benefit at 2 years for covered stents versus bare-metal stents in long SFA lesions: the VIASTAR Trial. Cardiovascular & Interventional Radiology 2015;38(1):25-32.
- Brodmann M. Real world value of the In.Pact Admiral DCB (Medtronic) for fem-pop lesions: from the In.Pact Global Registry: what else does it tell us. Presented at the 44th Annual Symposium on Vascular and Endovascular Issues, Techniques, Horizons (VEITHsymposium); November 14-18, 2017; New York, NY.
- Iida O, Nanto S, Uematsu M, Ikeoka K, Okamoto S, Nagata S. Influence of stent fracture on the long-term patency in the femoro-popliteal artery. JACC : Cardiovascular Interventions 2009;2(7):665-671.
- Scheinert D, Scheinert S, Sax J, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. Journal of the American College of Cardiology 2005;45(2):312-315.
- U.S. Food and Drug Administration. Center for Devices and Radiological Health. FDA Summary of Safety and Effectiveness Data. GORE TIGRIS Vascular Stent. P160004. http://www.accessdata.fda.gov/cdrh_docs/pdf16/P160004B.pdf. Published July 27, 2016. Accessed July 17, 2018.
- W. L. Gore & Associates, Inc. Evaluation of the GORE® TIGRIS® Vascular Stent in the Treatment of Atherosclerotic Lesions of the Superficial Femoral and Proximal Popliteal Arteries. [Final Post-Approval Study Report-Executive Summary]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2017. MD165299.
- Zeller T, Peeters P, Bosiers M, et al. Heparin-bonded stent-graft for the treatment of TASC II C and D femoropopliteal lesions: the Viabahn-25 cm Trial. Journal of Endovascular Therapy 2014;21(6):765-774.
- Reijnen M, van Walraven L, Fritschy W, et al. 1-year results of a multicenter, randomized controlled trial comparing heparin-bonded endoluminal to femoropopliteal bypass. Journal of Cardiovascular Interventions 2017;10(22):2320-2331.
- Ohki T, Kichikawa K, Yokoi H, et al. Long-term results of the Japanese multicenter Viabahn trial of heparin bonded endovascular stent grafts for long and complex lesions in the superficial femoral artery. Journal of Vascular Surgery. In press.
- Saxon RR, Chervu A, Jones PA, et al. Heparin‑bonded, expanded polytetrafluoroethylene‑lined stent graft in the treatment of femoropopliteal artery disease: 1‑year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) Trial. Journal of Vascular & Interventional Radiology 2013;24(2):165‑173.
- Iida O. Twelve-month outcomes from the Japanese post-market surveillance study of the GORE® VIABAHN® Endoprosthesis as treatment for symptomatic peripheral arterial disease in the superficial femoral arteries. Presented at LEIPZIG Interventional Course (LINC)2021; January 25-29, 2021; Leipzig, Germany.
- Iida O, Takahara M, Soga Y, et al; VANQUISH Investigators. One-year outcomes of heparin-bonded stent-graft therapy for real-world femoropopliteal lesions and the association of patency with the prothrombotic state based on the prospective, observational, multicenter Viabahn Stent-Graft Placement for Femoropopliteal Diseases Requiring Endovascular Therapy (VANQUISH) Study. Journal of Endovascular Therapy 2021;28(1):123-131.
- GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD174703.
- McQuade K, Gable D, Pearl G, Theune B, Black S. Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. Journal of Vascular Surgery 2010;52(3):584-591.
COOK® and ZILVER® PTX® are trademarks of COOK Medical, Inc.