GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement Data Review: Leaks
Protects against leaks
The only* staple line reinforcement proven to significantly reduce leaks in sleeve gastrectomy procedures.1
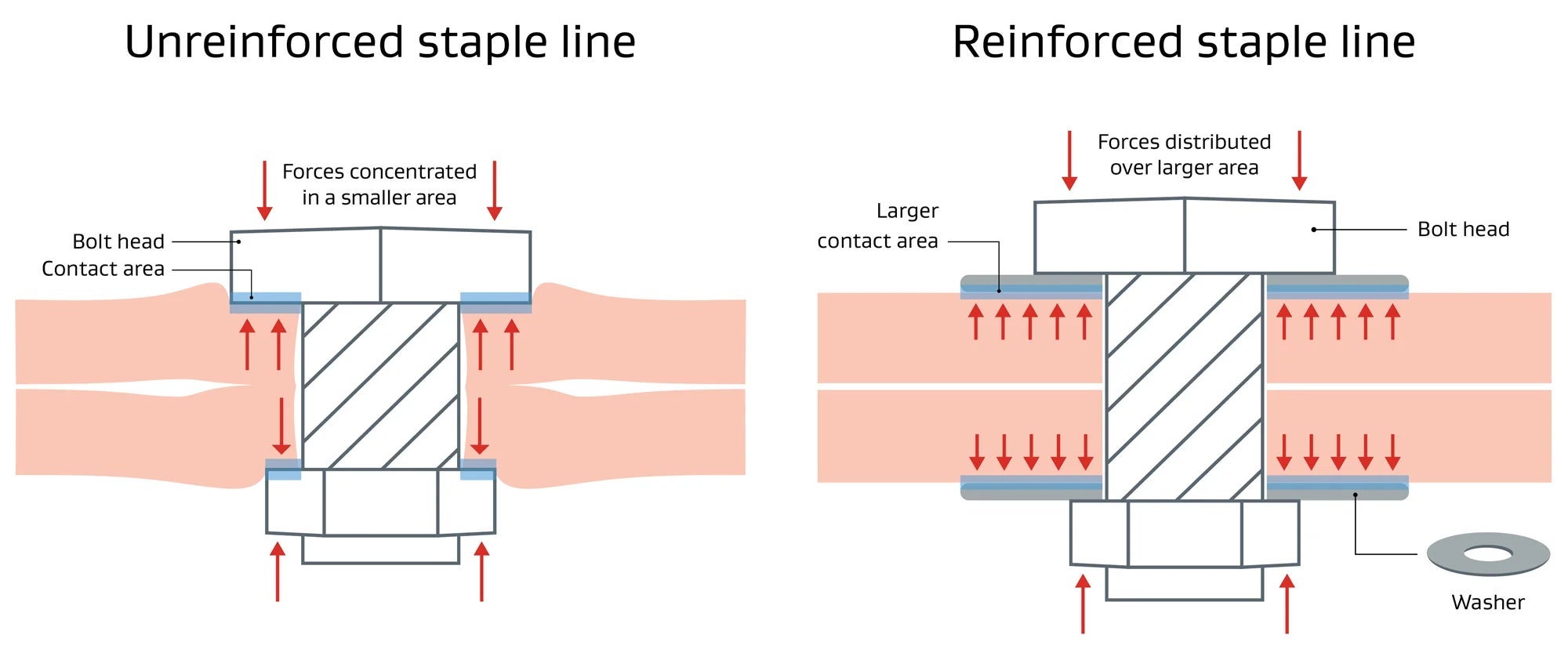
Pressure =
Force
Area
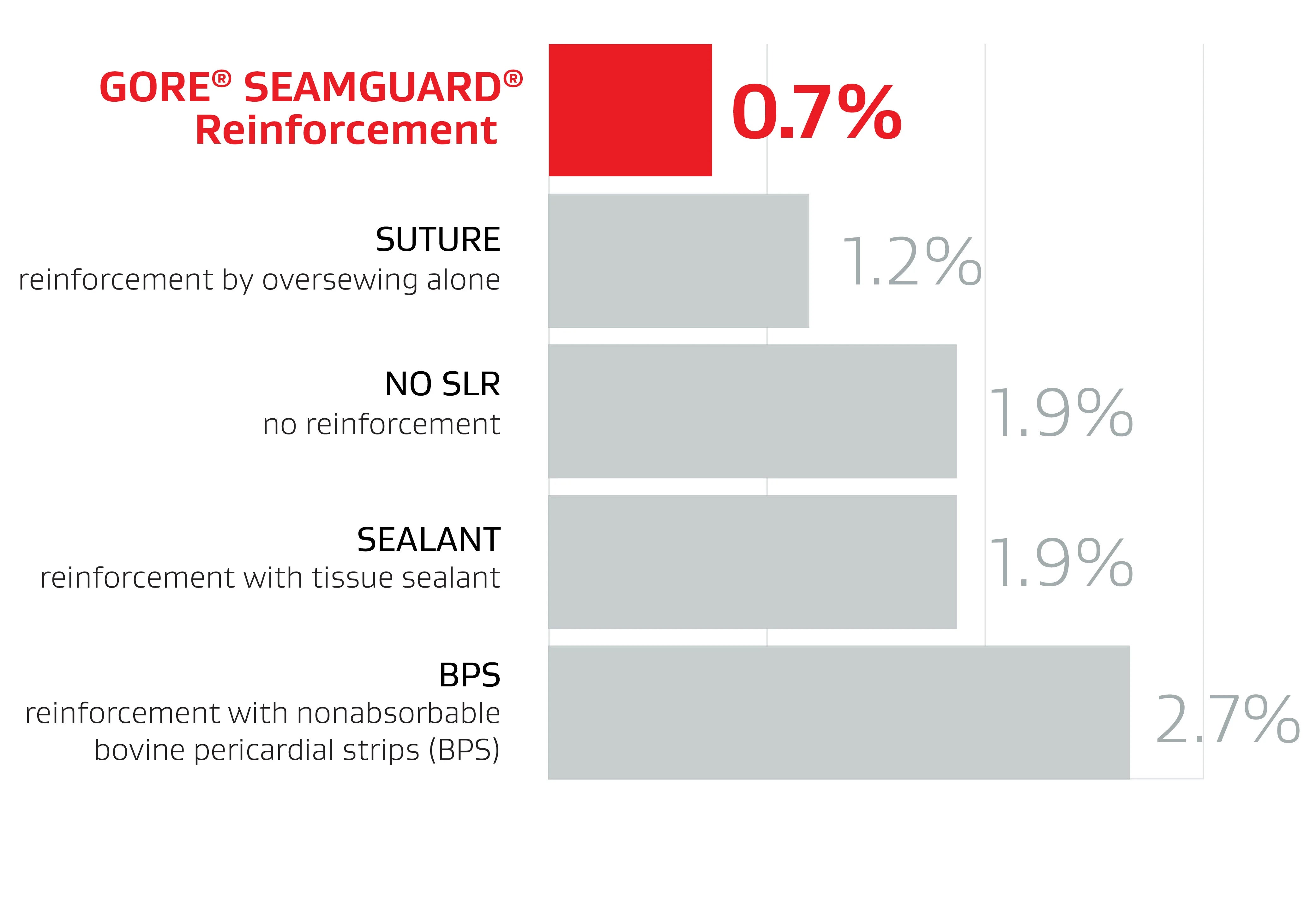
GORE® SEAMGUARD® Reinforcement had a significantly lower leak rate compared to other methods1
148 papers included in analysis
40,653 patients
* Considering all systematic review and meta-analysis of published articles only that distinguish between types of staple line reinforcement.
- Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surgical Endoscopy 2020;34(1):396-407.
- McCrea C. GORE® SEAMGUARD® Reinforcement Product Family: Relationship between Design Inputs and “Engineered to reduce the incidence of perioperative leaks and bleeding” Marketing Statement. Flagstaff, AZ; 2015. [Work plan]. WP107241

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which soft tissue transection or resection with staple line reinforcement is needed.
GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during lung resection, bronchial, bariatric, colon, colorectal, gastric, mesentery, pancreas and small bowel procedures.
CONTRAINDICATIONS: The GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is not indicated for patients requiring the patch reconstruction of cardiovascular defects such as cardiac, great vessel, and peripheral vascular arteries or veins.