Catalogue Numbers for GORE-TEX® STRETCH Vascular Graft For Vascular Access

Standard-walled | ||
|---|---|---|
| Catalogue Number | Internal Diameter (mm) | Standard Length (cm) |
| S0602E | 6 | 20 |
| S0603E | 6 | 30 |
| S0604E | 6 | 40 |
| S0704E | 7 | 40 |
| S0804E | 8 | 40 |
| S46045E* | 4-6 | 45 |
| S47030E* | 4-7 | 30 |
| S47045E* | 4-7 | 45 |
| S47055E* | 4-7 | 55 |
*Tapered
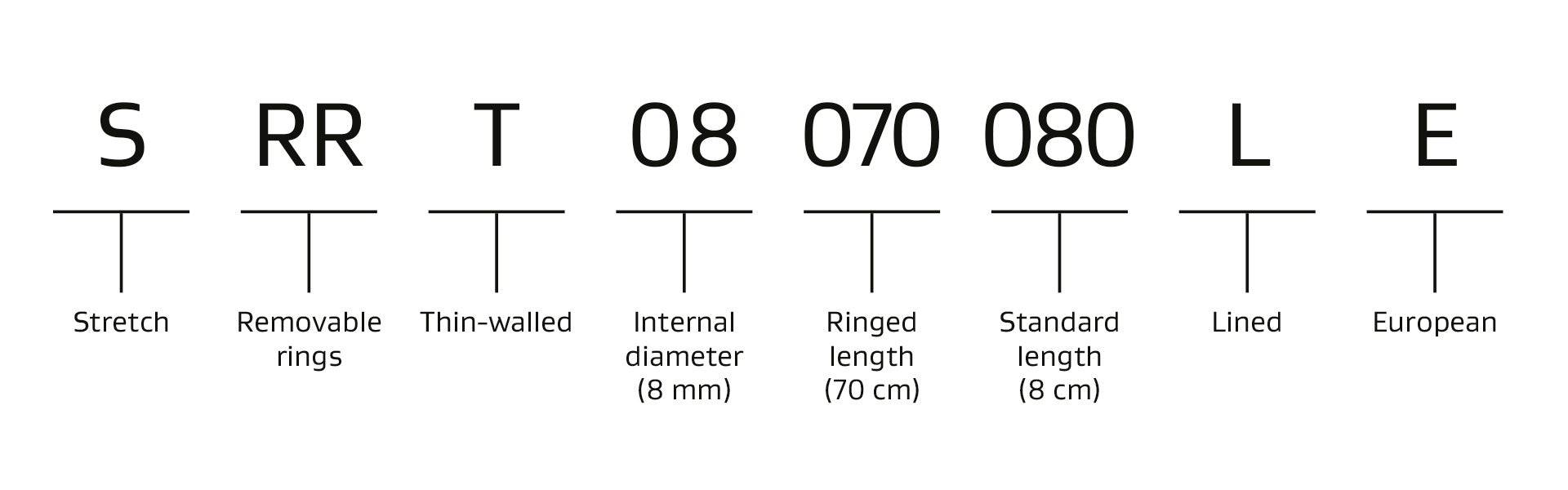
Sizing, availability and pricing varies by country.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: GORE® Vascular Graft is indicated for patients who require replacement or bypass of aortic or peripheral vessels due to occlusive or aneurysmal diseases or trauma, or for patients who require arteriovenous hemodialysis access in the upper or lower extremities due to end-stage renal disease (ESRD).
CONTRAINDICATIONS: DO NOT use GORE® Vascular Graft as a patch. If cut and used as a patch, GORE® Vascular Graft may lack adequate transverse strength. For cardiovascular procedures requiring patch materials, use the appropriate GORE‑TEX® Cardiovascular Patch.
DO NOT use any of the below configurations for coronary artery bypass or cerebral reconstruction procedures:
- GORE‑TEX® Vascular Graft FEP Ring,
- GORE‑TEX® Vascular Graft FEP Removable Ring,
- GORE‑TEX® STRETCH Vascular Graft FEP Removable Ring, or the
- GORE® INTERING® Vascular Graft.