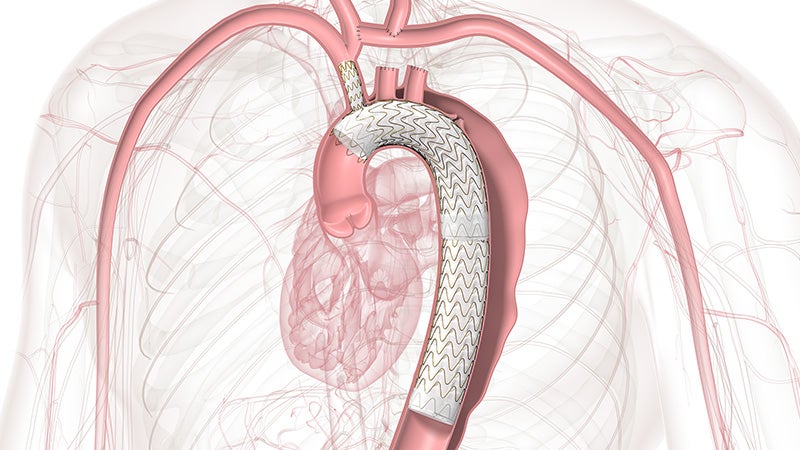
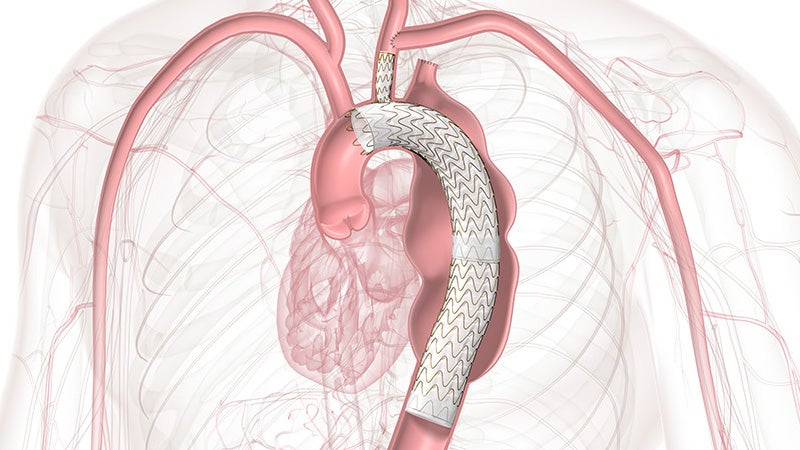
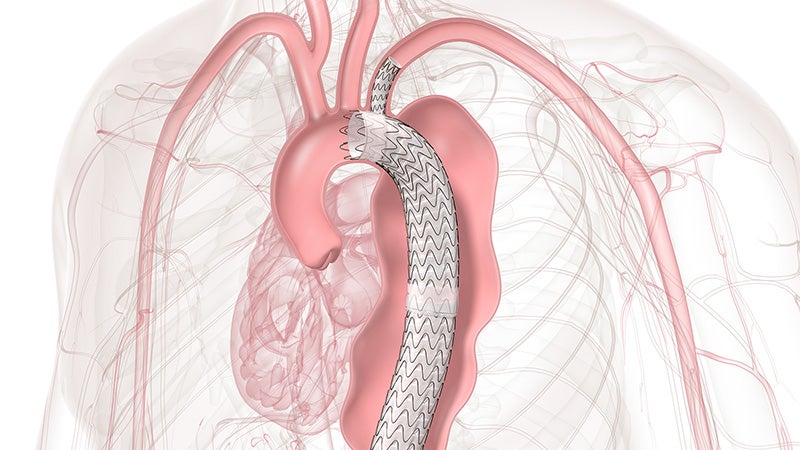
GORE® TAG® Thoracic Branch Endoprosthesis
A simplified solution within reach

Approved across all aortic arch zones
The off-the-shelf, single branch device physicians trust for Zone 2 branched TEVAR procedures is now also approved for Zones 0 and 1, expanding minimally invasive repair of all lesions involving the aortic arch.
Zones 0 and 1 approved
TBE gives physicians an endovascular, on-label option to perfuse a single target vessel, resulting in less procedural burden for Zones 0 and 1 by reducing the overall impact of procedures like sternotomy, cardiopulmonary bypass and circulatory arrest.
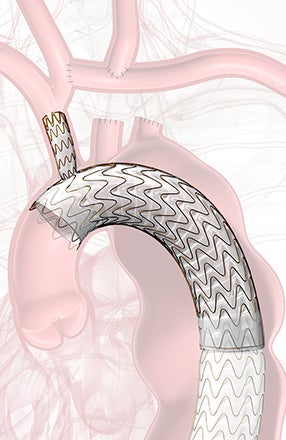
Zone 2 proven
TBE offers a demonstrated solution that preserves flow to the left subclavian artery (LSA) without the potential risks and complexity of surgical revascularization.

First-of-its kind FDA / MDR CE MARK approved device designed for simplified, minimally invasive Zone 2 TEVAR procedures. Deliver results without the potential risk and complexity of revascularization.
Off-the-shelf endovascular grafts are preferable to traditional surgical options for LSA preservation
Single Zone 2 endovascular procedure
5 days
average reduction in length of stay4
30 - 50 minutes
potential reduction in OR time4
How single-branch technology impacted a patient's life in emergency.
Staged open and TEVAR approach with TBE offers “complete and total thoracic solution” in multiple pathologies.
Whether it’s Zone 0, 1 or 2, consider TBE for your next aortic arch repair.
From pre-case planning to device procedural consultation, we are by your side to provide support.
- Training needs — Essential technical support with a deep reservoir of product knowledge.
- 8+ years — Average field representatives' tenure supporting Gore's aortic devices, building on diverse clinical backgrounds.
- Non-commissioned sales force — Our focus is on outcomes.

Related to this product:
a Did not require intervention.
b Core lab reported, 3 year (Total Through 36 Months(1-1275 days)).
c Embolization and thoracic stent graft placement were the reinterventions.
- Butterfield K, Hsu M, Powis S, Thon M. Evaluation of the GORE® TAG® Thoracic Branch Endoprosthesis (TBE Device) in the Treatment of Lesions of the Aortic Arch (Pivotal): Zone 0/1. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2024. [Pre-Market Approval Study Report]. MD200865. Rev 1.
- Data on file 2024; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- Brown J, Gorman J, Sondreaal M, Powis S. Evaluation of the GORE® TAG® Thoracic Branch Endoprosthesis (TBE Device) in the Treatment of Lesions of the Descending Thoracic Aorta (Pivotal): Zone 2. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. [SSB 11-02 Pivotal Pre-Market Approval Study Report]. MD185099. Rev 1.
- Squiers JJ, DiMaio JM, Schaffer JM, et al. Surgical debranching versus branched endografting in zone 2 thoracic endovascular aortic repair. Journal of Vascular Surgery 2022;75(6):1829-1836.e3.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® TAG® Thoracic Branch Endoprosthesis is intended for endovascular repair of all lesions of the descending thoracic aorta (including isolated lesions, such as aneurysm and traumatic transection, and Type B dissections) while maintaining flow into the left subclavian artery, in patients who have appropriate anatomy.
CONTRAINDICATIONS: The GORE® TAG® Thoracic Branch Endoprosthesis is contraindicated in: Patients with known sensitivities or allergies to the device materials [ePTFE (polytetrafluoroethylene), FEP (Fluoroethylpropylene), Nitinol (Nickel, Titanium), Gold, SB Component only - Heparin (CBAS® Heparin surface]; Patients who have a condition that threatens to infect the graft; Patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.