Catalogue Numbers for GORE® VIABIL® Biliary Endoprosthesis for Endoscopic and Percutaneous delivery

GORE® VIABIL® Biliary Endoprosthesis Endoscopic Delivery System
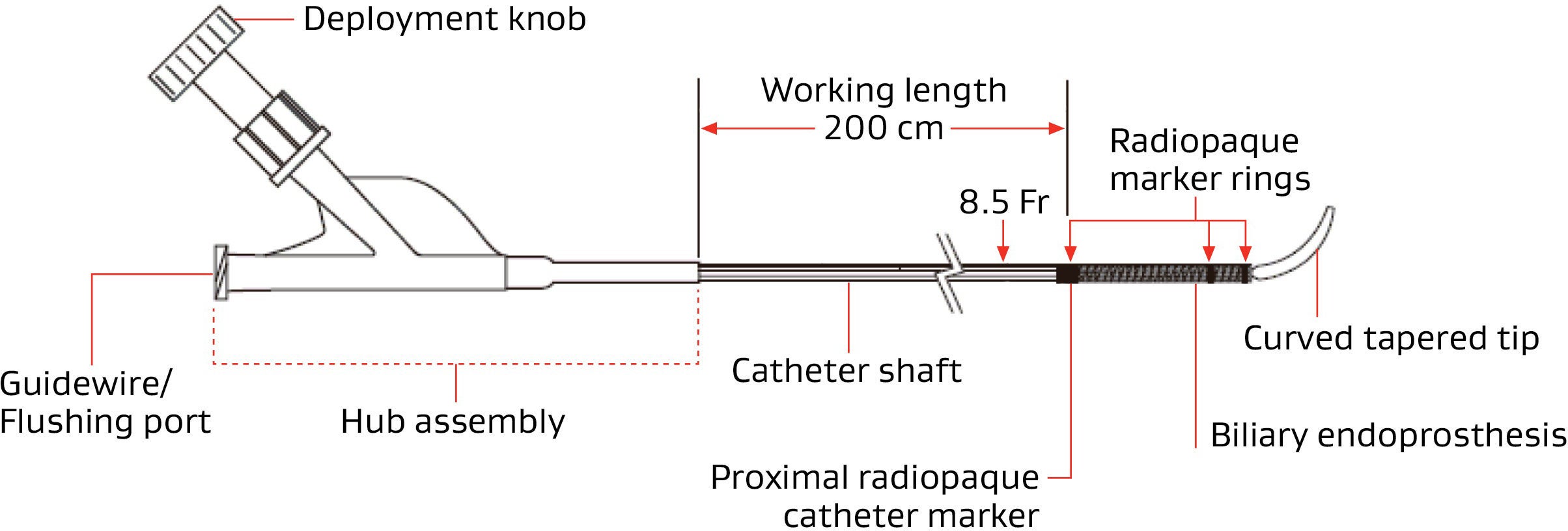
GORE® VIABIL® Short Wire Biliary Endoprosthesis Endoscopic Delivery System
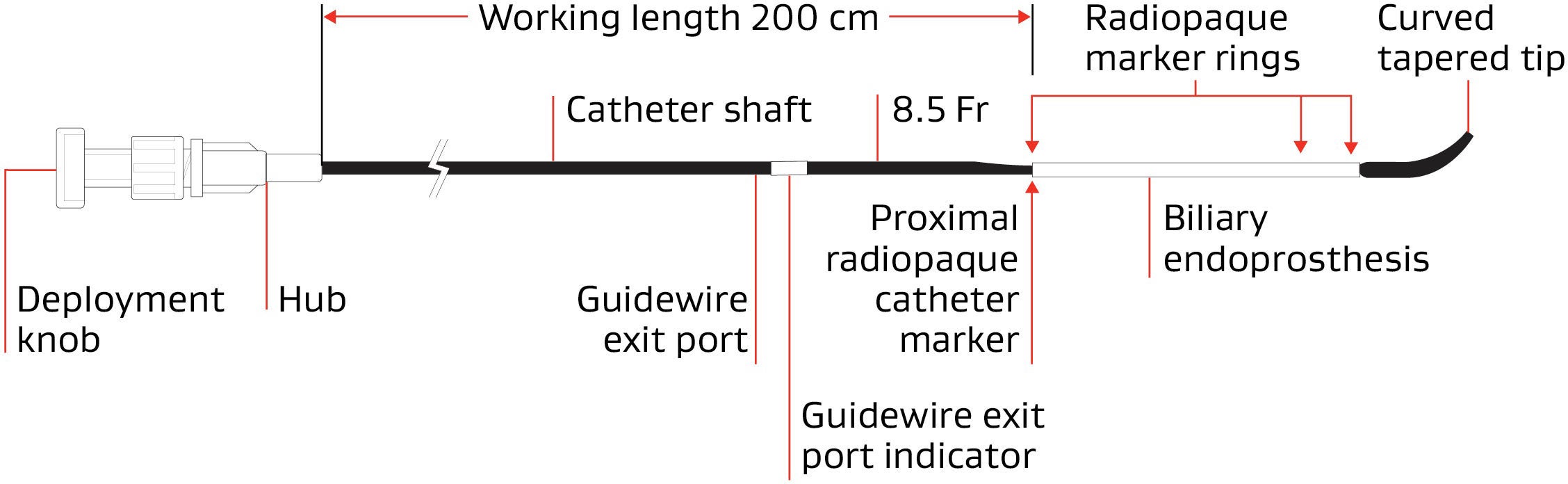
Endoscopic delivery | |||||
|---|---|---|---|---|---|
GORE® VIABIL® Biliary Endoprosthesis Catalogue Number | GORE® VIABIL® Short Wire Biliary Endoprosthesis Catalogue Number | Nominal Endoprosthesis Diameter (mm) × Length (cm) | Working Length of Delivery Catheter (cm) | Drainage Holes Located at the Hilar Region | Transmural Drainage Holes Length (cm) |
| VN0804200R | VSWVN0804R | 8 × 4 | 200 | No holes | - |
| VN0806200R | VSWVN0806R | 8 × 6 | 200 | No holes | - |
| VN0808200R | VSWVN0808R | 8 × 8 | 200 | No holes | - |
| VN0810200R | VSWVN0810R | 8 × 10 | 200 | No holes | - |
| VN1004200R | VSWVN1004R | 10 x 4 | 200 | No holes | - |
| VN1006200R | VSWVN1006R | 10 x 6 | 200 | No holes | - |
| VN1008200R | VSWVN1008R | 10 x 8 | 200 | No holes | - |
| VN1010200R | VSWVN1010R | 10 x 10 | 200 | No holes | - |
| VH0806200 | VSWVH0806 | 8 x 6 | 200 | Holes | 2 |
| VH0808200 | VSWVH0808 | 8 x 8 | 200 | Holes | 2 |
| VH0810200 | VSWVH0810 | 8 x 10 | 200 | Holes | 2 |
| VH1006200 | VSWVH1006 | 10 x 6 | 200 | Holes | 2 |
| VH1008200 | VSWVH1008 | 10 x 8 | 200 | Holes | 2 |
| VH1010200 | VSWVH1010 | 10 x 10 | 200 | Holes | 2 |
GORE® VIABIL® Biliary Endoprosthesis Percutaneous Delivery System
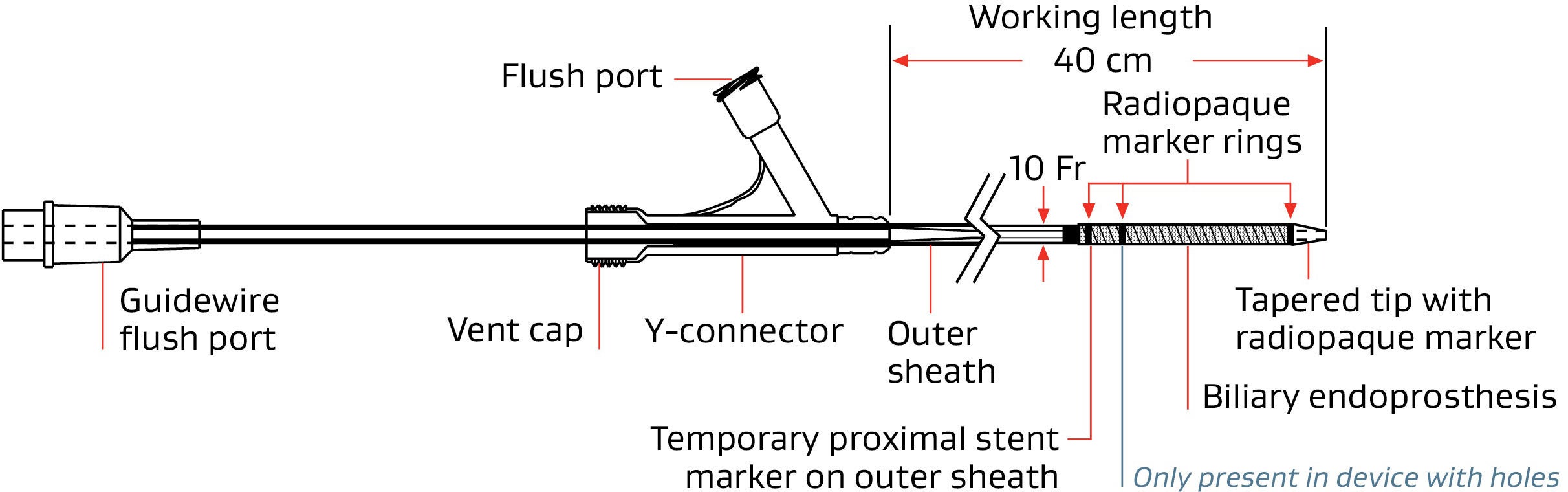
Percutaneous delivery | |||
|---|---|---|---|
GORE® VIABIL® Biliary Endoprosthesis Catalogue Number | Nominal Endoprosthesis Diameter (mm) × Length (cm) | Working Length of Delivery Catheter (cm) | Drainage Holes Located at the Hilar Region |
| VN0804040R | 8 × 4 | 40 | No holes |
| VN0806040R | 8 × 6 | 40 | No holes |
| VN0808040R | 8 × 8 | 40 | No holes |
| VN0810040R | 8 x 10 | 40 | No holes |
| VN1004040R | 10 × 4 | 40 | No holes |
| VN1006040R | 10 × 6 | 40 | No holes |
| VN1008040R | 10 × 8 | 40 | No holes |
| VN1010040R | 10 × 10 | 40 | No holes |
| VH0806040 | 8 × 6 | 40 | Holes |
| VH0808040 | 8 × 8 | 40 | Holes |
| VH0810040 | 8 x 10 | 40 | Holes |
| VH1006040 | 10 × 6 | 40 | Holes |
| VH1008040 | 10 × 8 | 40 | Holes |
| VH1010040 | 10 × 10 | 40 | Holes |
Sizing, availability and pricing varies by country.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE:
FOR ENDOSCOPIC DELIVERY
INDICATIONS:
(Non-Removable) The GORE® VIABIL® Biliary Endoprosthesis is indicated for palliation of malignant strictures in the biliary tree.
(Removable): The Removable GORE® VIABIL® Biliary Endoprosthesis is indicated for the treatment of benign and malignant biliary strictures and can be removed from such strictures for up to one year post implant.
CONTRAINDICATIONS:
(Non-Removable): The GORE® VIABIL® Biliary Endoprosthesis is contraindicated for: ALL CARDIOVASCULAR APPLICATIONS; ducts less than 5.5 mm in diameter or greater than 9 mm in diameter.
(Removable): The Removable GORE® VIABIL® Biliary Endoprosthesis is contraindicated for: ALL CARDIOVASCULAR APPLICATIONS; ducts less than 5.5 mm in diameter or greater than 9 mm in diameter; removal when positioned within a previously placed bare metal stent.
FOR GORE® VIABIL® SHORT WIRE BILIARY ENDOPROSTHESIS FOR ENDOSCOPIC DELIVERY
INDICATIONS:
(Non-Removable) The GORE® VIABIL® Short Wire Biliary Endoprosthesis is indicated for palliation of malignant strictures in the biliary tree.
(Removable): The Removable GORE® VIABIL® Short Wire Biliary Endoprosthesis is indicated for the treatment of benign and malignant biliary strictures and can be removed from such strictures for up to one year post implant.
CONTRAINDICATIONS:
(Non-Removable): The GORE® VIABIL® Short Wire Biliary Endoprosthesis is contraindicated for: ALL CARDIOVASCULAR APPLICATIONS; ducts less than 5.5 mm in diameter or greater than 9 mm in diameter.
(Removable): The Removable GORE® VIABIL® Short Wire Biliary Endoprosthesis is contraindicated for: ALL CARDIOVASCULAR APPLICATIONS; ducts less than 5.5 mm in diameter or greater than 9 mm in diameter.
FOR PERCUTANEOUS DELIVERY
INDICATIONS:
(Non-Removable) The GORE® VIABIL® Biliary Endoprosthesis is indicated for palliation of malignant strictures in the biliary tree.
(Removable): The Removable GORE® VIABIL® Biliary Endoprosthesis is indicated for the treatment of benign and malignant biliary strictures and can be removed from such strictures for up to one year post implant.
CONTRAINDICATIONS:
(Non-Removable): The GORE® VIABIL® Biliary Endoprosthesis is contraindicated for: ALL CARDIOVASCULAR APPLICATIONS; ducts less than 5.5 mm in diameter or greater than 9 mm in diameter.
(Removable): The Removable GORE® VIABIL® Biliary Endoprosthesis is contraindicated for: ALL CARDIOVASCULAR APPLICATIONS; ducts less than 5.5 mm in diameter or greater than 9 mm in diameter; removal when positioned within a previously placed bare metal stent.