Restoring flow to a brachioaxillary AV graft after multiple failed PTA revisions
A case study using the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*
Challenge
- 48-year-old male with end stage renal disease secondary to hypertensive nephrosclerosis and diabetic nephropathy
- Relevant patient history:
- Diabetes mellitus, chronic anemia, paroxysmal atrial fibrillation, coronary artery disease, hypertension, hyperlipidemia, COPD, tobacco use
- Left brachioaxillary arteriovenous (AV) graft 4-7 mm implanted June 26, 2018. Two revisions, April 8, 2019 and August 23, 2019, each with an BD® ULTRASCORE® Focused Forced PTA Balloon followed by a 9 mm x 40 mm BD® LUTONIX® 035 Drug Coated Balloon PTA Catheter
- Relevant patient history:
- Presented after two failed percutaneous transluminal angioplasty (PTA) revisions of the venous anastomosis of an arteriovenous graft, preventing successful hemodialysis.
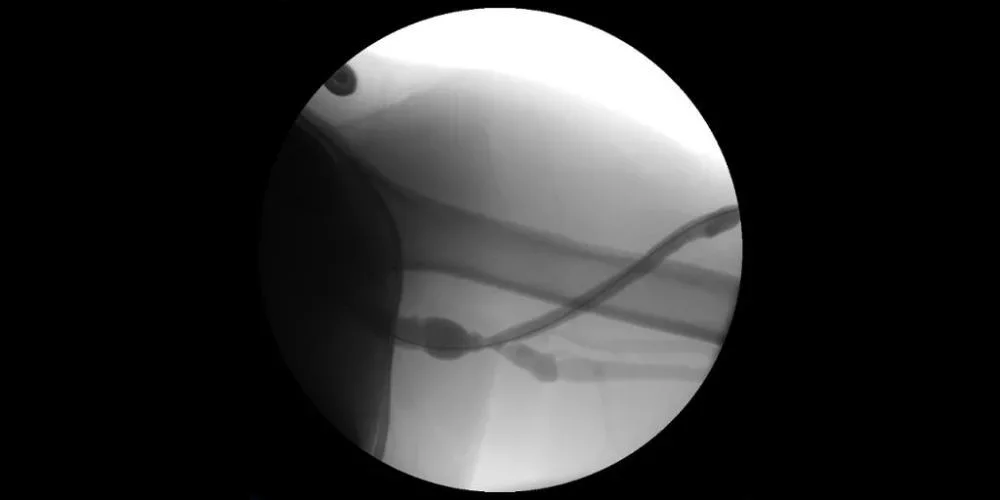
Image: Fistulography with recurrent stenosis noted at venous outflow anastomosis.
Images courtesy of Nicolas Mouawad, M.D. Used with permission.
Procedure
- Planned definitive treatment with outflow stenting
- Advanced the TERUMO® RADIFOCUS® GLIDEWIRE® ADVANTAGE .035" Guidewire across the target lesion (Left image)
- Pre-dilated the stenotic lesion with an 8 x 40 mm MEDTRONIC EVERCROSS PTA BALLOON Catheter (Left image)
- Placed a 9 mm x 5 cm .035" guidewire compatible VIABAHN® Device (Right image)
- Post-dilated with a 9 x 40 mm MEDTRONIC EVERCROSS PTA BALLOON Catheter PTA (Right image)
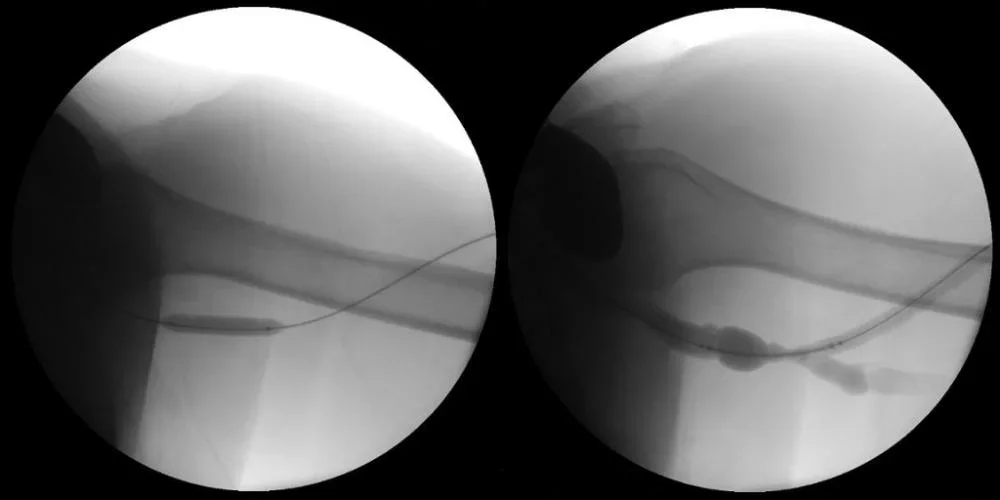
Left image: Lesion preparation with pre-dilatation using 8 x 40 mm noncompliant balloon.
Right image: Post-PTA outflow fistulography in preparation for VIABAHN® Device deployment.
Images courtesy of Nicolas Mouawad, M.D. Used with permission.
Result
Excellent outflow without any recurrent complication at eight months post-intervention.
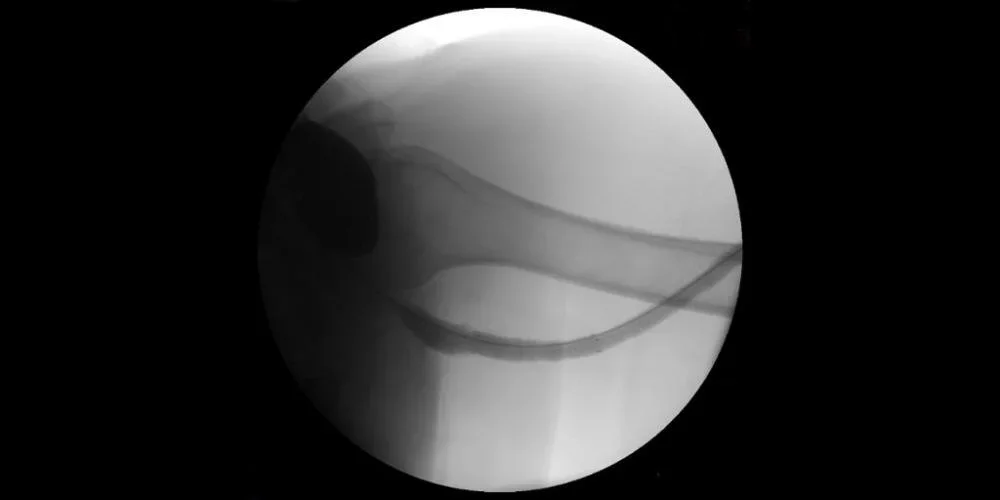
Image: Fistulogram following stent deployment with complete resolution of stenosis.
Images courtesy of Nicolas Mouawad, M.D. Used with permission.
Case takeaways
The outcome of this case aligns to the well-established findings that the VIABAHN® Device offers value through reduced frequency of repeat interventions as compared to PTA.1
Enhanced access and visibility in challenging anatomies is enabled by a low profile and radiopaque markers on the proximal and distal ends of the device.
At eight months post-placement, the VIABAHN® Device has exceeded results achieved with prior PTA treatments; primary patency of the stent graft is maintained, with no circuit reinterventions post VIABAHN® Device placement.

Related case studies
* As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
- Mohr BA, Sheen AL, Roy-Chaudhury P, Schultz SR, Aruny JE; REVISE Investigators. Clinical and economic benefits of stent grafts in dysfunctional and thrombosed hemodialysis access graft circuits in the REVISE Randomized Trial. Journal of Vascular & Interventional Radiology 2019;30(2):203-211.e4. https://www.jvir.org/article/S1051-0443(18)31772-X/fulltext
BD, LUTONIX and ULTRASCORE are trademarks of Becton, Dickinson and Company.
MEDTRONIC and EVERCROSS are trademarks of Medtronic, Inc.
TERUMO and RADIFOCUS are trademarks of Terumo Medical Corporation.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use (IFU) or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.

