GORE® PROPATEN® Vascular Graft Clinical Data: Below-knee data
GORE® PROPATEN® Vascular Graft Below-knee bypass primary patency
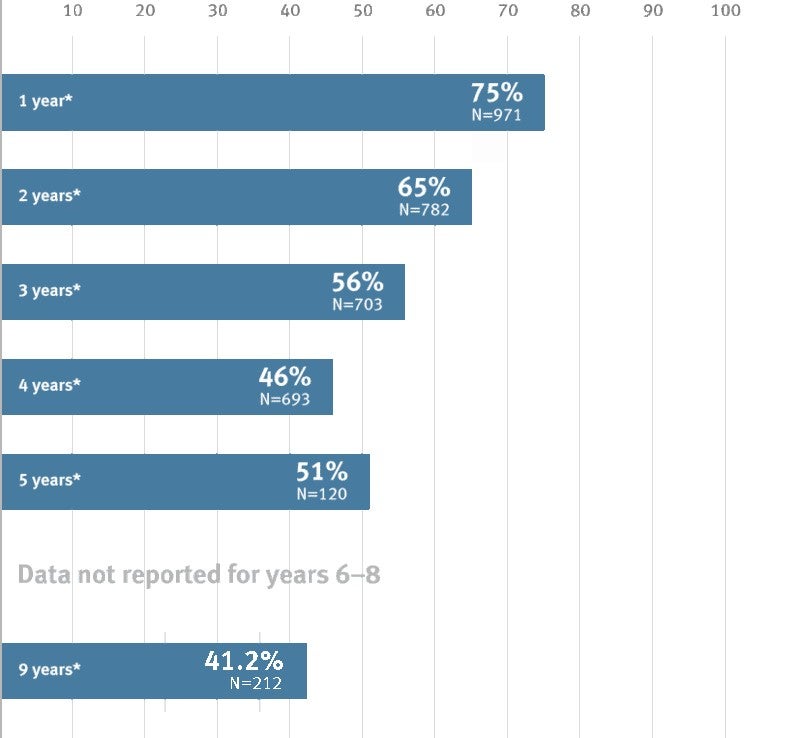
* Overall weighted average primary patency is based on data from 10 peer-reviewed publications meeting pre-determined inclusion criteria.
Study size (N) reflects the initial cohort size of the study
Below-knee data | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Location | N | Primary Patency | |||||
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 9 | ||||
| Kaisar et al | 2018 | Infrapopliteal | 62 | 85% | 71% | 64% | 57% | ||
| Uhl et al | 2015 | Infrapopliteal | 89 | 64% | 49% | 34% | |||
| Neville et al | 2014 | Infrapopliteal | 62 | 75% | |||||
| Monaca et al | 2013 | Infrapopliteal | 212 | 52% | 41% | ||||
| Dorigo et al | 2012 | BK fem-pop Infrapopliteal | 414 142 | 45% 39% | |||||
| Pulli et al | 2010 | BK fem-pop Infrapopliteal | 238 86 | 75% 66% | 67% 57% | 61% 52% | |||
| Daenens et al | 2009 | BK fem-pop Infrapopliteal | 57 97 | 92% 79% | 83% 69% | ||||
| Hugl et al | 2009 | BK fem-pop Infrapopliteal | 37 15 | 74% 79% | |||||
| Lösel-Sadée et al | 2009 | BK fem-pop Infrapopliteal | 30 45 | 77% 64% | 71% 57% | 71% 50% | 71% 50% | 71% 50% | |
| Peeters et al | 2008 | BK fem-pop Infrapopliteal | 41 37 | 86% 71% | 79% 60% | 75% 60% | |||
Inclusion criteria
Inclusion criteria for below-knee, fem-pop and infrapopliteal GORE® PROPATEN® Vascular Graft data used in this analysis:
- Peer-reviewed clinical journal publications in English language
- Non-overlapping patient populations
- BK bypass primary patency reported for at least 12 months of follow-up
- N = 50 or more BK bypass (BK fem-pop and BK fem-distal) in the GORE® PROPATEN® Vascular Graft treatment arm

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: GORE® PROPATEN® Vascular Graft is indicated for patients who require replacement or bypass of peripheral vessels due to occlusive or aneurysmal diseases or trauma, or for patients who require arteriovenous hemodialysis access in the upper or lower extremities due to end-stage renal disease (ESRD).
GORE® PROPATEN® Vascular Graft Configured for Pediatric Shunt is indicated for staged palliative repair of cyanotic congenital heart disease (CCHD).
CONTRAINDICATIONS: DO NOT use the GORE® PROPATEN® Vascular Graft in patients with known hypersensitivity to heparin, including those patients who have had a previous incidence of Heparin-Induced Thrombocytopenia (HIT) type II.
DO NOT use any of the below configurations of GORE® PROPATEN® Vascular Graft for coronary artery bypass or cerebral reconstruction procedures:
- GORE® PROPATEN® Vascular Graft Integrated Rings
- GORE® PROPATEN® Vascular Graft Fixed Ring
- GORE® PROPATEN® Vascular Graft Removable Ring
- GORE® PROPATEN® Vascular Graft Removable Ring Axillobifemoral
DO NOT use GORE® PROPATEN® Vascular Graft as a patch. If cut and used as a patch, GORE® PROPATEN® Vascular Graft may lack adequate transverse strength.
FOR PATCHING APPLICATIONS: For cardiovascular procedures requiring patch materials, use the appropriate GORE® ACUSEAL Cardiovascular Patch.