
Positive outcomes and reducing costs for complex hernia repairs
The GORE® BIO-A® Tissue Reinforcement provides cost saving benefits and quality patient outcomes. It provides proven performance with an innovative material that has consistent absorption, leaving no permanent material behind in the body.
This material can be used in complex applications where biologics would be chosen but without the risks or concerns around the use of biologic materials. GORE® BIO-A® Tissue Reinforcement offers proven performance at considerable economic value.
Positive outcomes and reducing costs for complex hernia repairs
GORE® BIO-A® Tissue Reinforcement was used as an alternative to current synthetic material where a full-absorbable material is desired. Here are the results:
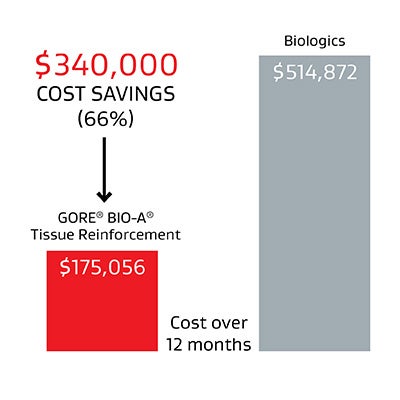
66%
savings over biologics*
realized by leading midwest medical center over a 12-month period when using GORE® BIO-A® Tissue Reinforcement instead of biologics.
The Future of Value Analysis
A Handbook for Health Care Professionals
Read perspectives from value analysis professionals who share their thoughts regarding the importance of effective collaboration, paradigm shifts with determining value, and the critical focus on the future of healthcare.

* Data on file 2017, W. L. Gore & Associates, Inc., Flagstaff, AZ.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE: The GORE® BIO-A® Tissue Reinforcement is intended for use in the reinforcement of soft tissue. An example of an application where the GORE® BIO-A® Tissue Reinforcement may be used is hernia repair as suture line reinforcement.
CONTRAINDICATIONS: The GORE® BIO-A® Tissue Reinforcement is contraindicated for use in reconstruction of cardiovascular defects. Because GORE® BIO-A® Tissue Reinforcement is absorbable, it is contraindicated for use in patients requiring permanent support from the device.
