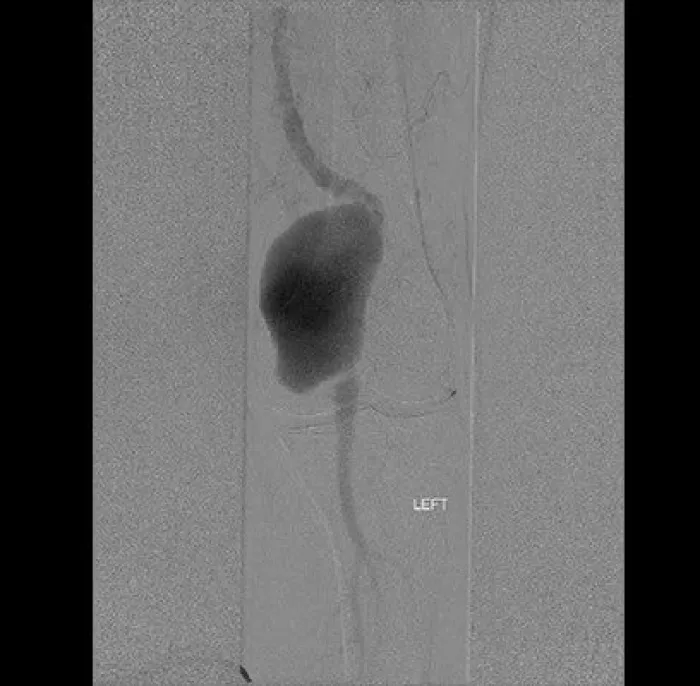
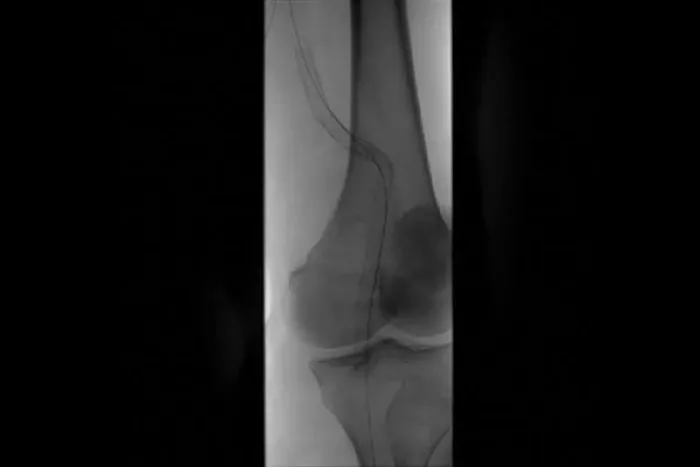
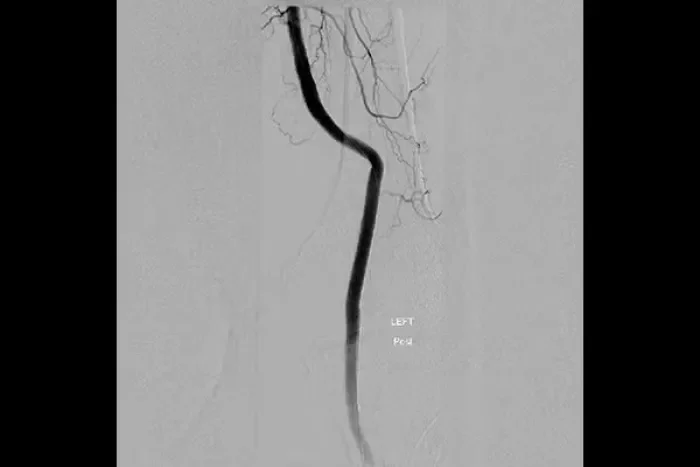
Popliteal artery aneurysms
Endovascular treatment of popliteal artery aneurysms (PAAs) is associated with reduced operative time, perioperative morbidity, hospital stay, and recovery time compared to open surgery.1-7
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is an established PAA treatment option associated with short and mid-term clinical results that are comparable to surgical bypass.2-7
Proven Patency
Two to six year primary patency in PAA of 70 – 86% which is comparable to those reported for surgical bypass at five years (69 – 88%)2-7
Demonstrated Durability
10-year primary, primary assisted, and secondary patency rates in PAA of 51%, 57%, and 60%, respectively1
- Golchehr B, Zeebregts CJ, Reijnen MMPJ, Tielliu IFJ. Long-term outcome of endovascular popliteal artery aneurysm repair. Journal of Vascular Surgery. 2018; 67(6): 1797-1804
- Antonello M, Frigatti P, Battocchio P, et al. Endovascular treatment of asymptomatic popliteal aneurysm: 8-year concurrent comparison with open repair. Journal of Cardiovascular Surgery 2007;48(3):267-274.
- Curi MA, Geraghty PJ, Merino OA, et al. Mid-term outcomes of endovascular popliteal artery aneurysm repair. Journal of Vascular Surgery 2007;45(3):505-510.
- Ghotbi R, Sotiriou A, Schönhofer S, Zikos D, Schips K, Westermeier W. Stent-graft placement in popliteal artery aneurysms: midterm results. Vascular Disease Management 2007;4(4):123-127.
- Rajasinghe HA, Tzilinis A, Keller T, Schafer J, Urrea S. Endovascular exclusion of popliteal artery aneurysms with expanded polytetrafluoroethylene stent-grafts: early results. Vascular & Endovascular Surgery 2007;40(6):460-466.
- Tielliu IFJ, Verhoeven ELG, Zeebregts CJ, Prins PR, Bos WTGJ, van den Dungen JJAM. Endovascular treatment of popliteal artery aneurysms: is the technique a valid alternative to open surgery? Journal of Cardiovascular Surgery 2007;48(3):275-279
- Wain RA, Hines G. A contemporary review of popliteal artery aneurysms. Cardiology in Review 2007;15(2):102-107

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
Product may not be available in all countries. Please check with your Gore representative for availability.