Long lesions of the superficial femoral artery (SFA) present complex challenges for endovascular management
As lesion length increases, treatment outcomes typically worsen, with decreased patency demonstrated in studies of drug-eluting stents, drug-coated balloons and bare metal stents.1-7
Proven outcomes in long SFA lesions
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* has demonstrated excellent patency and durability independent of lesion length.4, 8-11
Peer-Reviewed Results
Access the published study outcomes in Vascular Medicine, the official journal of the Society for Vascular Medicine.
Objective and methodology
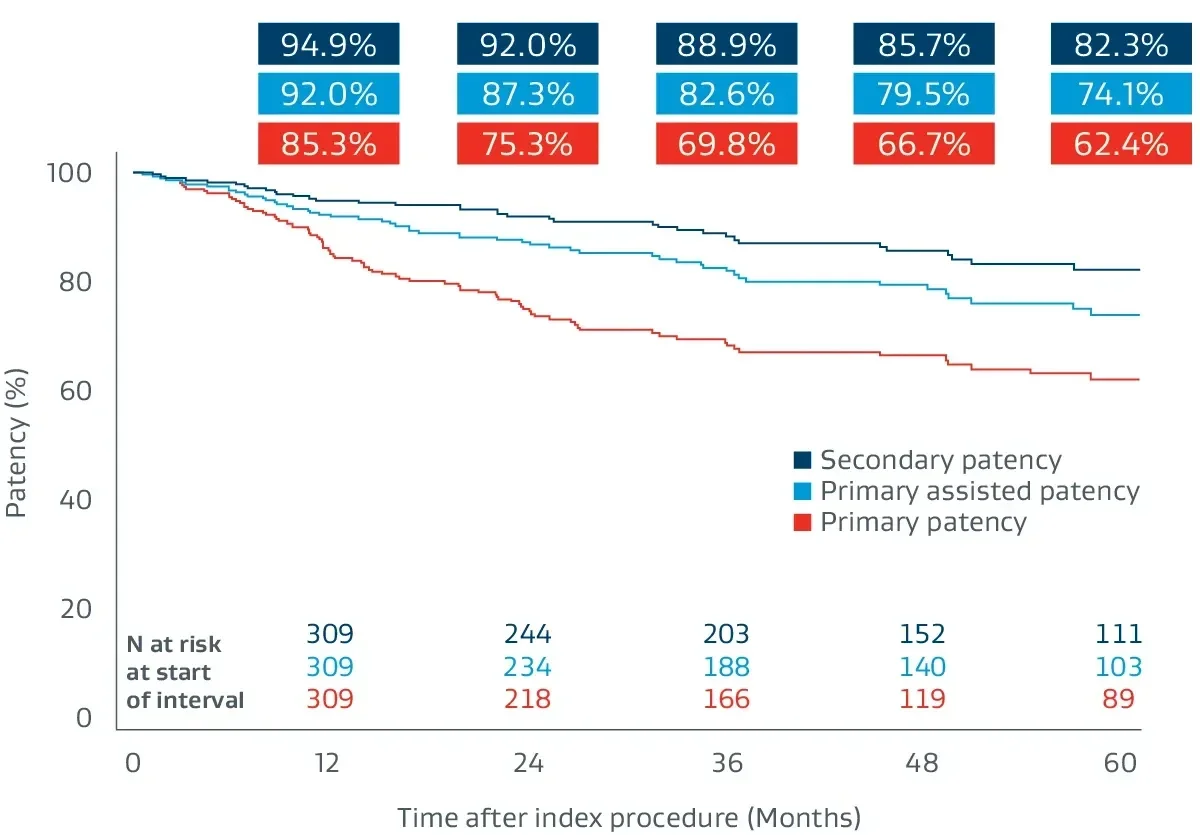
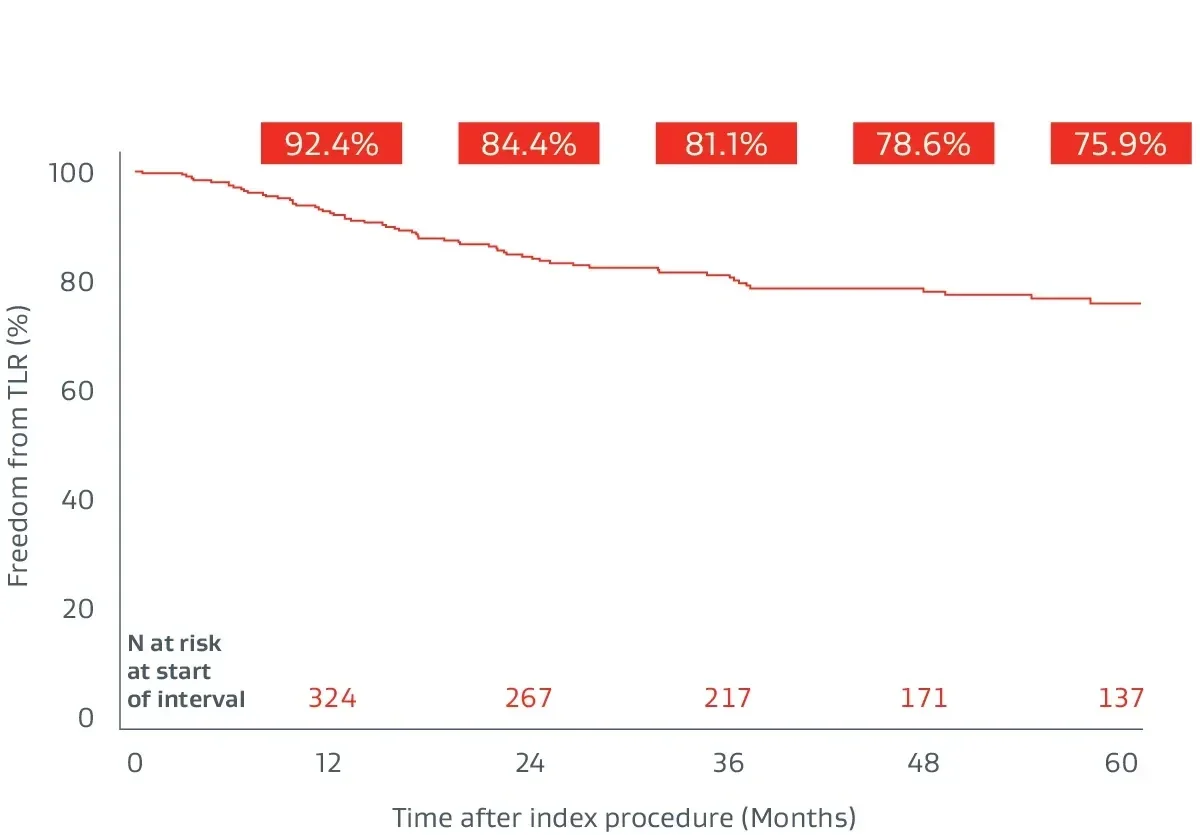
Prospective, multicenter (64 sites), 5-year follow-up builds on 1-year post-market surveillance.12, 13
- 324 lesions, 24 cm average lesion length
- 70% chronic total occlusions (CTOs)
- 27% critical limb-threatening ischemia (CLTI)
- 48% TASC II D lesions
Durable clinical outcomes
The following outcomes were first presented during the late-breaking clinical trials session at VIVA 2023.
See the results
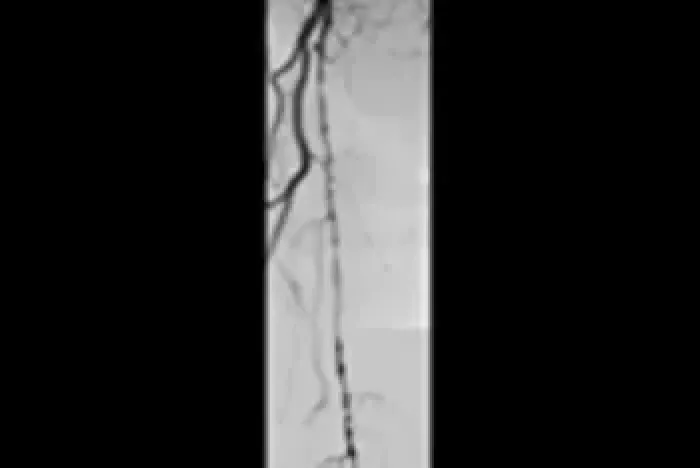
Six-year follow-up imaging shows freedom from re-occlusion despite significant popliteal lesion progression caused by chronic atherosclerotic disease.
Initial Angiograms
Prior to treatment with the VIABAHN® Device
Proximal SFA
Distal SFA
Six-year follow-up angiograms
Patency maintained with the VIABAHN® Device
Proximal SFA
Distal SFA
Images courtesy of Osamu Iida, M.D. Used with permission.
Extending a robust evidence base
Proven patency in complex SFA lesions across 7 multicenter, prospective, randomized or single-arm studies.4,8-12,14
1,089
lesions studied
71%
chronic total occlusions (CTO)
23 cm
average lesion length‡
80%
average primary patency§
Consider the demands of treating long-length lesions of the SFA
- Lesion length is a predictor of patency outcomes for several treatment modalities.
- Drug-eluting stents
- Lesion length has been shown to be an independent predictor of restenosis.1
- In a randomized study, COOK® ZILVER® PTX® Drug-Eluting Peripheral Stent has been shown to have lower patency in long lesions > 10 cm compared to those 10 cm or shorter.2
- Lesion length has been shown to be an independent predictor of restenosis.1
- Drug coated balloon (DCB)
- Multivariate analysis of the Medtronic IN.PACT Global Study demonstrated that increasing lesion length was a predictor of increased risk for Clinically Driven Target Lesion Revascularization (CD TLR).3
- Multivariate analysis of the Medtronic IN.PACT Global Study demonstrated that increasing lesion length was a predictor of increased risk for Clinically Driven Target Lesion Revascularization (CD TLR).3
- Bare metal stents (BMS)
- The VIASTAR Trial demonstrated BMS had lower patency when treating lesions > 20 cm than when treating lesions shorter than 20 cm.4
- The VIASTAR Trial demonstrated BMS had lower patency when treating lesions > 20 cm than when treating lesions shorter than 20 cm.4
- Drug-eluting stents
- Complications increase with lesion length
- Risk of dissection when using DCB increases with lesion length.5
- For many BMS, the risk of stent fracture increases with lesion length and is associated with a loss of patency.6,7
- Risk of dissection when using DCB increases with lesion length.5
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface
‡ Weighted average lesion length.
§ One-year weighted average primary patency.
1. Iida O, Takahara M, Soga Y, et al; ZEPHYR Investigators. 1-year results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): predictors of restenosis. JACC: Cardiovascular Interventions 2015;8(8):1105-1112.
2. Bausback Y. 2 year results of the REAL PTX Randomized Clinical Trial comparing Zilver PTX DES vs. DCB in femoropopliteal lesions. Presented at the 7th Munich Vascular Conference (MAC); December 7-9, 2017; Munich, Germany.
3. Micari A, Brodmann M, Keirse K, et al; IN.PACT Global Study Investigators. Drug-coated balloon treatment of femoropopliteal lesions for patients with intermittent claudication and ischemic rest pain: 2-year results from the IN.PACT Global Study. JACC: Cardiovascular Interventions 2018;11(10):945-953.
4. Lammer J, Zeller T, Hausegger KA, et al. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). Journal of the American College of Cardiology 2013;62(15):1320-1327.
5. Brodmann M. Real world value of the In.Pact Admiral DCB (Medtronic) for fem-pop lesions: from the In.Pact Global Registry: what else does it tell us. Presented at the 44th Annual Symposium on Vascular and Endovascular Issues, Techniques, Horizons (VEITHsymposium); November 14-18, 2017; New York, NY.
6. Iida O, Nanto S, Uematsu M, Ikeoka K, Okamoto S, Nagata S. Influence of stent fracture on the long-term patency in the femoro-popliteal artery. JACC : Cardiovascular Interventions 2009;2(7):665-671.
7. Scheinert D, Scheinert S, Sax J, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. Journal of the American College of Cardiology 2005;45(2):312-315.
8. Zeller T, Peeters P, Bosiers M, et al. Heparin-bonded stent-graft for the treatment of TASC II C and D femoropopliteal lesions: the Viabahn-25 cm Trial. Journal of Endovascular Therapy 2014;21(6):765-774.
9. Reijnen MMPJ, van Walraven LA, Fritschy WM, et al. 1-year results of a multicenter, randomized controlled trial comparing heparin-bonded endoluminal to femoropopliteal bypass. Journal of Cardiovascular Interventions 2017;10(22):2320-2331.
10. Ohki T, Kichikawa K, Yokoi H, et al. Long-term results of the Japanese multicenter Viabahn trial of heparin bonded endovascular stent grafts for long and complex lesions in the superficial femoral artery. Journal of Vascular Surgery 2021;74(6):1958-1967.e2. https://www.sciencedirect.com/science/article/pii/S0741521421010119
11. Saxon RR, Chervu A, Jones PA, et al. Heparin‑bonded, expanded polytetrafluoroethylene‑lined stent graft in the treatment of femoropopliteal artery disease: 1‑year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) Trial. Journal of Vascular & Interventional Radiology 2013;24(2):165‑173.
12. Iida O, Ohki T, Soga Y, et al. Twelve-month outcomes from the Japanese post-market surveillance study of the Viabahn Endoprosthesis as treatment for symptomatic peripheral arterial disease in the superficial femoral arteries. Journal of Endovascular Therapy 2022;29(6):855-865.
13. Iida O, Ohki T, Soga Y, et al. Five-Year outcomes of the GORE VIABAHN Endoprosthesis for the treatment of complex femoropopliteal lesions from a Japanese post-market surveillance study. Vascular Medicine 2024;29(4):416-423.
14. Iida O, Takahara M, Soga Y, et al; VANQUISH Investigators. One-year outcomes of heparin-bonded stent-graft therapy for real-world femoropopliteal lesions and the association of patency with the prothrombotic state based on the prospective, observational, multicenter Viabahn Stent-Graft Placement for Femoropopliteal Diseases Requiring Endovascular Therapy (VANQUISH) Study. Journal of Endovascular Therapy 2021;28(1):123-131.
15. GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface Instructions for Use (IFU). W. L. Gore & Associates, Inc. Accessed August 9, 2023. https://eifu.goremedical.com
COOK and ZILVER PTX are trademarks of Cook Medical, Inc.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.