Product indications and/or availability may vary by region. Please consult the product's Instructions for Use for your region for complete information, including indications, contraindications, warnings and precautions.
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* for treating in-stent restenosis (ISR) of the superficial femoral artery (SFA)
Decide with data
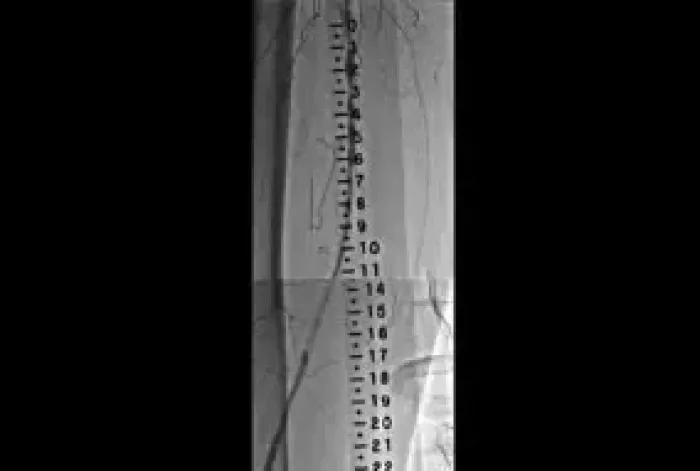
Three-year study results from the Gore RELINE MAX Clinical Study demonstrate durable outcomes in the treatment of real-world SFA ISR lesions.
This data was first presented during the late-breaking clinical trials session at VIVA 2022.
Objective and methodology1
- Single-arm, prospective, multicenter study (23 U.S. sites and 3 European sites)
- 86 patients analyzed (Per-protocol)
- Real-world ISR lesion presentation:
- 52% long diffuse lesions
- 29% total occlusions
- 33% moderate/severe calcification
- 52% long diffuse lesions
PROVEN EFFICACY THROUGH 3 YEARS1:
65%
freedom from target lesion revascularization
>80%
of patients maintained ≥ 1 Rutherford category improvement
.24
improvement in mean resting ankle-brachial index (ABI)
(P < .001, .92 mean ABI)‡
PROVEN SAFETY THROUGH 3 YEARS1:
100%
freedom from major amputation§
100%
freedom from VIABAHN® Device stent fractures
Consider the complexity and challenges of treating SFA ISR:
- Case complexities such as calcification, total occlusions and long lesions have been associated with an increased rate of restenosis, occlusion and provisional stenting in SFA disease.2-7
- ISR is the “Achilles’ heel” of peripheral vascular interventions.8
- Physicians estimate 63% of ISR lesions are long and diffuse or total occlusions while 35% have a moderate to high degree of calcification.9
- Physicians estimate 63% of ISR lesions are long and diffuse or total occlusions while 35% have a moderate to high degree of calcification.9
- Reintervention frequency is cited as a top ISR treatment challenge, with physicians estimating the average patient experiences 7 ISR reinterventions over 3 years.9
* As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
‡ (P < .001) Statistically significant change from preprocedure.
§ In a cohort including Rutherford category 4+ patients at baseline.
- Soukas P, Becker M, Stark K, Tepe G; RELINE MAX Investigators. Three-year results of the GORE® VIABAHN® endoprosthesis in the superficial femoral artery for in-stent restenosis. Journal of the Society for Cardiovascular Angiography & Interventions 2023;2(6)Part A:101183. OPEN ACCESS https://www.sciencedirect.com/science/article/pii/S2772930323011857
- Kum S. Evolution and challenges of ISR. How to approach. Presented at Leipzig Interventional Course (LINC 2020); January 28-31, 2020; Leipzig, Germany.
- Tepe G, Laird J, Schneider P, et al; IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA Randomized Trial. Circulation 2015;131(5):495-502.
- Iida O, Takahara M, Soga Y, et al; ZEPHYR Investigators. 1-year results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): predictors of restenosis. JACC: Cardiovascular Interventions 2015;8(8):1105-1112.
- Kistner CM, Lammer J, Willfort-Ehringer A, et al. Paclitaxel-eluting balloon versus standard balloon angioplasty in in-stent restenosis of the superficial femoral and proximal popliteal artery:1-year results of the PACUBA Trial. JACC: Cardiovascular Interventions 2016;9(13):1386-1392.
- Varghese V, Virk HUH, Lakhter V, et al. Femoral artery chronic total occlusion revascularization (FACTOR) score and algorithm: feasibility and validation in a single-center study of femoropopliteal occlusions. Journal of Invasive Cardiology 2020;32(12):E338-E348.
- Scheinert D, Micari A, Brodmann M, et al; IN.PACT Global Study Investigators. Drug-coated balloon treatment for femoropopliteal artery disease. Circulation:Cardiovascular Interventions 2018;11(10):e005654.
- Adams GL, Bersin RM, George JC, Subramanian V, Soukas PA. Data-driven treatment approach to in-stent restenosis. Endovascular Today 2015;14(6) Supplement:3-8.
- W. L. Gore & Associates Inc. Market Assessment for ISR in SFA Procedures- Global Research. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2022. [Market research]. 22715502-EN.
- Bosiers M, Deloose K, Callaert J, et al. Stent-grafts are the best way to treat complex in-stent restenosis lesions in the superficial femoral artery: 24-month results from a multicenter randomized trial. The Journal of Cardiovascular Surgery 2020;61(5):617-25.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.