

Proven material that provides economic value
GORE® ENFORM Biomaterial devices are intended for use in the reinforcement of soft tissue during the phases of wound healing by filling soft-tissue deficits. The device is completely absorbed, leaving no material behind in the body.
As an alternative to expensive biologics, GORE® ENFORM Biomaterials support savings in the high-cost category of plastic and reconstructive surgery.*
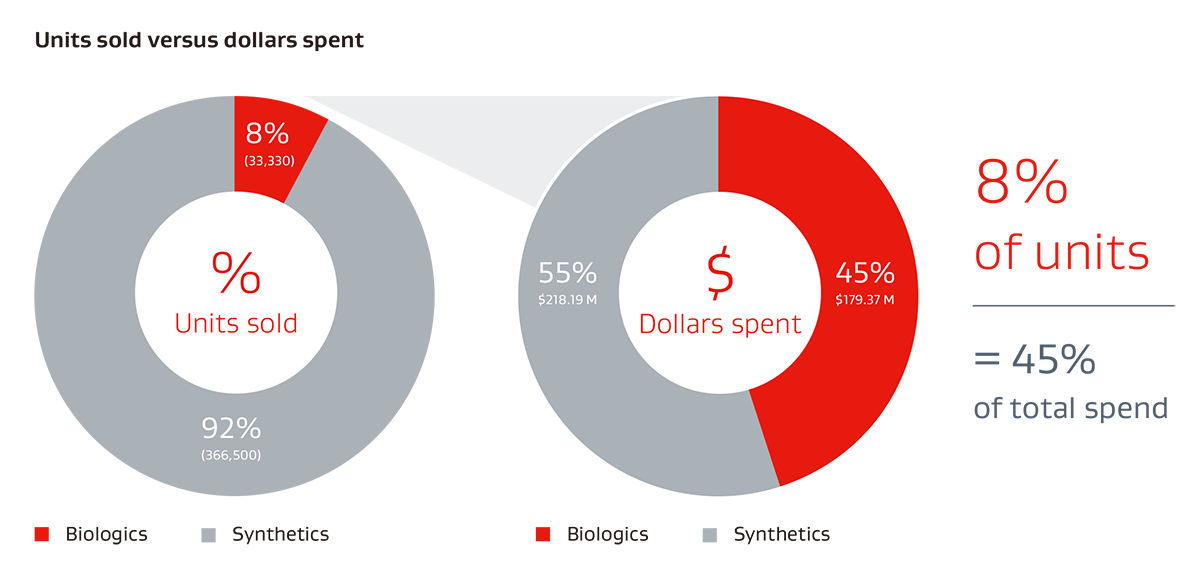
Typical ventral hernia mesh spend for U.S. hospitals 20191
Economic impact
14
Surgical procedures with
GORE® ENFORM Biomaterials
= $74,172
Savings realized†
$530k
Estimated annual savings for this academic center when choosing GORE® ENFORM Biomaterials over biologics, with 100 cases
The Future of Value Analysis
A Handbook for Health Care Professionals
Read perspectives from value analysis professionals who share their thoughts regarding the importance of effective collaboration, paradigm shifts with determining value, and the critical focus on the future of healthcare.

* Examples of applications where the GORE® ENFORM Biomaterials may be used include hernia repair as suture-line reinforcement, muscle flap reinforcement and general tissue reconstructions.
† Data on file 2019; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- © 2022 Millennium Research Group, Inc. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE: The GORE® ENFORM Intraperitoneal Biomaterial and GORE® ENFORM Preperitoneal Biomaterial are indicated for use in the reinforcement of soft tissue. This includes use in patients requiring soft tissue reinforcement in plastic and reconstructive surgery. Examples of applications where the GORE® ENFORM Biomaterial may be used include hernia repair as suture-line reinforcement, muscle flap reinforcement and general tissue reconstructions.
CONTRAINDICATIONS: The GORE® ENFORM Intraperitoneal Biomaterial and GORE® ENFORM Preperitoneal Biomaterial are contraindicated for use in reconstruction of cardiovascular defects. Because GORE® ENFORM Biomaterial is absorbable, it is contraindicated for use in patients requiring permanent support from the device.
