Education

Evolving Our Education Offerings to Meet Your Needs
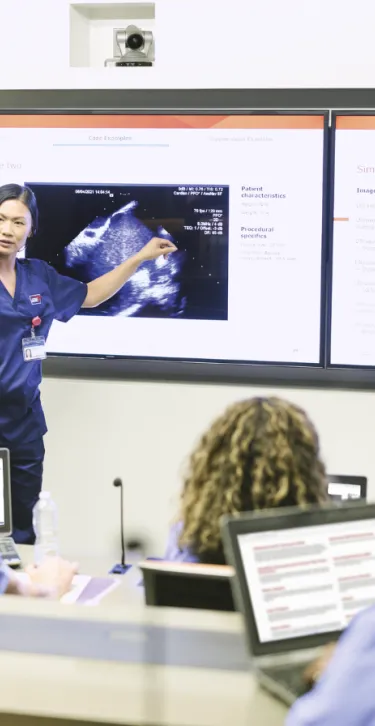
At Gore, we are committed to empowering health care professionals through comprehensive education programs. Whether you are looking for in-person or online offerings, our blended learning approach is designed to equip you with the knowledge and tools you need to enhance patient outcomes.
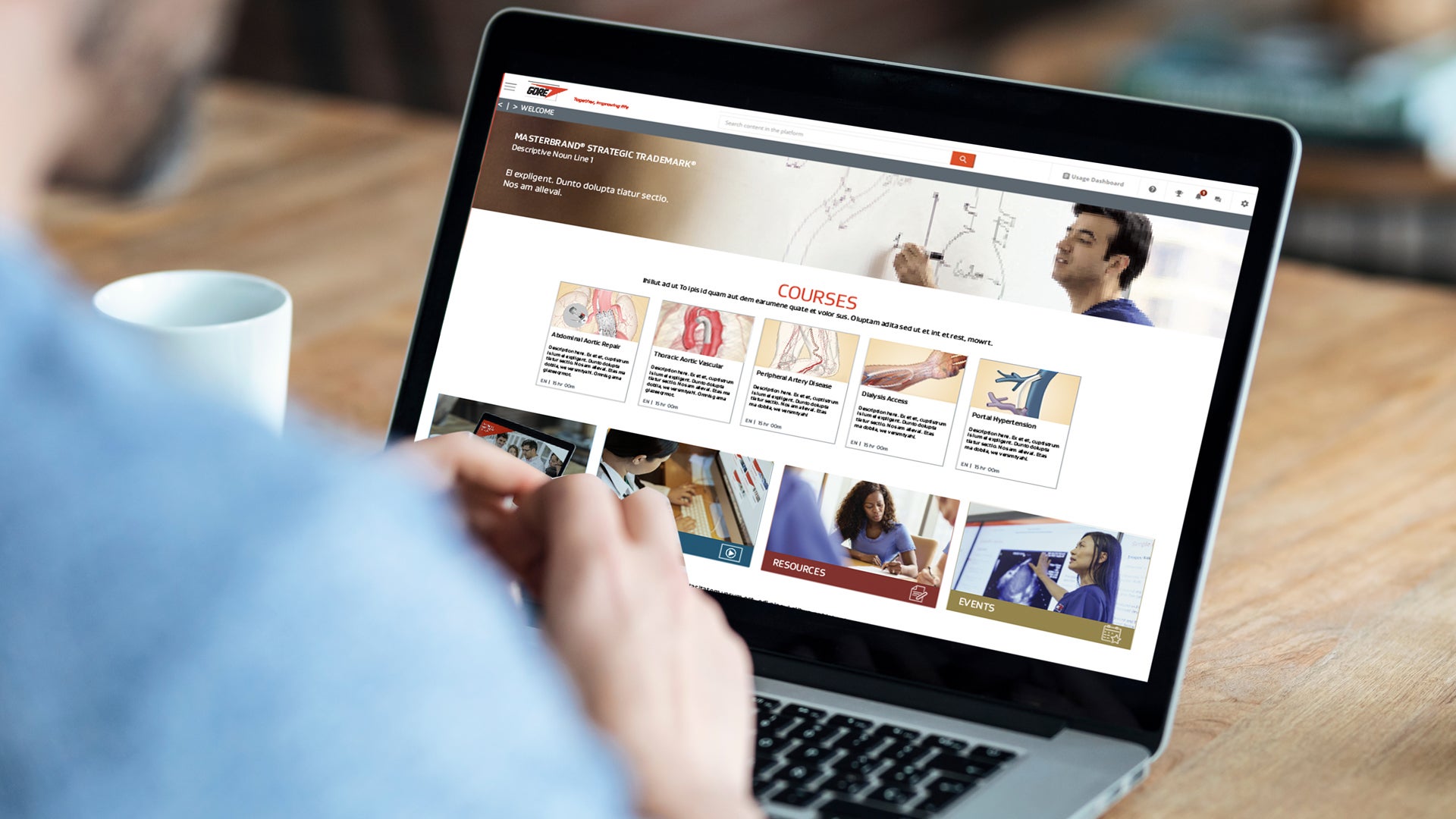
Gore Learn360
Gore Learn360 is an online education platform providing clinical product training designed to help health care professionals elevate their practice in the field. This centralized hub delivers a rich and accessible integrated learning experience through video, animation, text and interactive content, ranging from deployment and technique demonstrations to informative case studies, linking to blended and in-person educational opportunities.
Case Study: Treating a Type B Aortic Dissection (TBAD)
GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL
24MD1080-EN01


