Treatment of a large popliteal artery aneurysm
Challenge
The patient is a 67-year-old male with a history of hypertension, dyslipidemia and coronary artery disease. He underwent two vessel coronary artery bypass graft surgeries in 1998. He underwent left nephrectomy due to renal carcinoma. He presented in the office complaining of pain in his left leg with ambulation as well as a pulsatile mass behind the left knee. The patient denied any history of smoking or excessive alcohol intake. Exam was notable for diminished pulses in the left foot and a pulsatile mass palpable behind the left knee. Current medications include amlodipine / benazepril, metoprolol, rosuvastatin, aspirin and clopidogrel. Lower extremity Doppler exam revealed mildly reduced ABI of 0.9 in the left leg compared to 1.1 in the right leg. There was a large popliteal aneurysm in the left leg measuring 5.0 cm with mild aneurysmal dilatation noted in the right popliteal artery. The study was otherwise unremarkable. In view of his severe coronary artery disease which was complicated by late graft closure, the patient was felt to be high risk for surgical aneurysm repair and exclusion of the graft using a percutaneous approach was recommended.
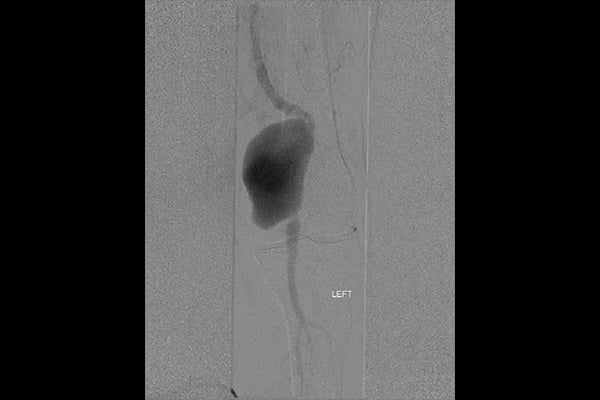
Image: Large (5 cm diameter) left popliteal artery aneurysm.
Images courtesy of Barry S. Weinstock, M.D. Used with permission.
Procedure
Angiography was performed using a contralateral approach with right common femoral access. A 5 Fr Universal Flush diagnostic catheter was used for aorto-iliac angiography and was then advanced over the aortoiliac bifurcation to the left common femoral artery to perform selective left lower extremity digital subtraction angiography. This demonstrated normal arterial circulation except for a large left popliteal aneurysm with swirling blood flow within the aneurysm and an angulated vessel kink in the SFA just proximal to the aneurysm. The diagnostic catheter and sheath were then exchanged for a 7 Fr Terumo PINNACLE® DESTINATION® Contralateral Sheath. The patient was minimally anti-coagulated with heparin 2500 units IV. A 0.018” 300 cm SV5 guide wire (Boston Scientific) was carefully steered through the aneurysm using a 5 Fr 100 cm Terumo GLIDECATH® Angled Glide Catheter. The SV5 wire was advanced to the distal peroneal artery. The popliteal aneurysm was initially excluded using a low-profile, 0.018” compatible 7 x 150 mm GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*. This placement also avoided overlap or landing zones near the major hinge point of the popliteal artery, just proximal to the knee joint. After high pressure balloon angioplasty of the Endoprosthesis with a 7 x 80 mm SUBMARINE PLUS Angioplasty Balloon (Medtronic Invatec), the patient exhibited small but definite proximal and distal endoleaks. The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* was therefore extended distally using a low-profile 7 x 50 mm device and extended proximally using a low profile 8 x 50 mm device. The overlapped Endoprostheses were dilated at high pressure using an 8 x 40 mm STERLING® SL Balloon Catheter (Boston Scientific Corp.).
Result
Final angiography showed complete aneurysm exclusion with no endoleak and brisk flow to the foot with no evidence of distal embolization. The patient was discharged on the same day as the procedure. At office follow-up, the patient is asymptomatic and the pulsatile mass behind the left knee is no longer palpable. Distal pulses in the left leg are now strong and equal to the pulses in the right leg. A follow-up Doppler study demonstrated complete exclusion of the aneurysm and no evidence of endoleak.
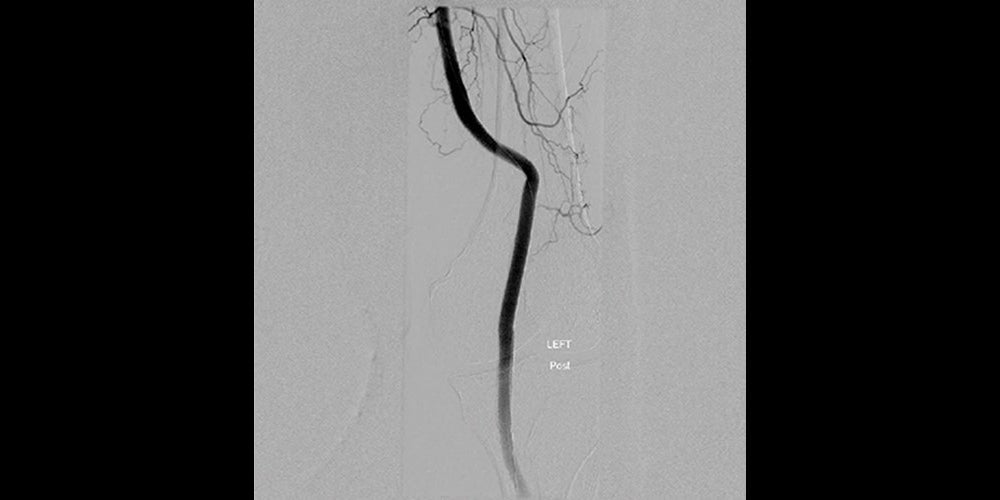
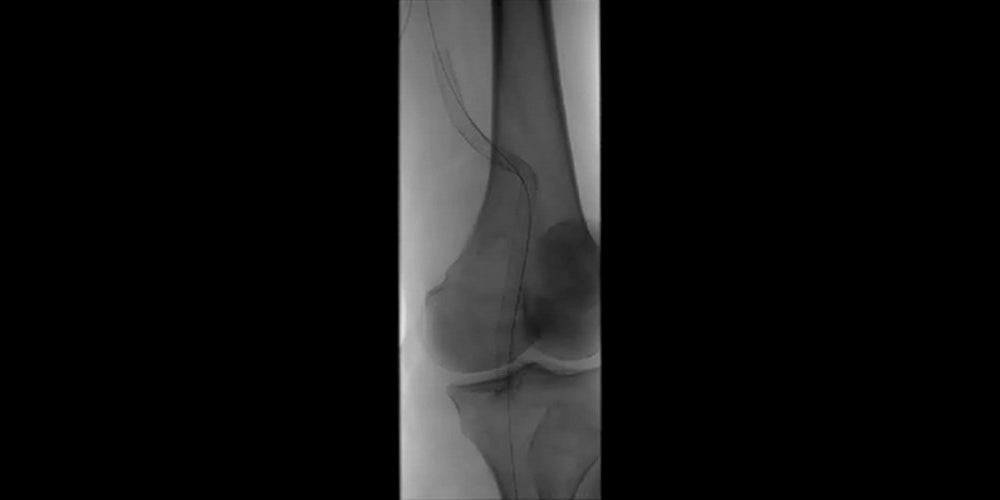
Images (from left to right): Stent-graft placement to exclude the aneurysm; Completion angiography showing good conformability to the artery tortuosity and total exclusion of the aneurysm by GORE® VIABAHN® Devices.
Images courtesy of Barry S. Weinstock, M.D. Used with permission.
Case takeaways
The new, low-profile 0.018” compatible GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* is ideal for treatment of popliteal aneurysms. The use of the 0.018” wire and the lower profile GORE® VIABAHN® Delivery System minimized the risk of distal embolization of thrombus within the aneurysm. Further, the lower profile system allowed the procedure to be performed through a 7 Fr contralateral sheath rather than an 8 Fr sheath which would have been required for both the 7 mm and 8 mm 0.035”-compatible GORE® VIABAHN® Devices. The new, low profile GORE® VIABAHN® Delivery System not only minimized the chances of distal embolization, it also reduced the risk of access site bleeding complications by allowing use of a smaller introducer sheath. The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* is an excellent alternative to surgical repair of popliteal aneurysms and offers lower morbidity as well as a shorter length of stay.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of Healthcare Providers. Healthcare Providers remain solely responsible for making decisions about patient care and the use of medical technologies.
* PROPATEN Bioactive Surface is synonymous with the CBAS Heparin Surface.
DESTINATION®, GLIDECATH® and PINNACLE® are trademarks of Terumo Medical Corporation.
SUBMARINE PLUS is a trademark of Invatec.
STERLING® SL is a trademark of Boston Scientific Corporation.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.