Product Value Summary
GORE® CARDIOFORM ASD Occluder

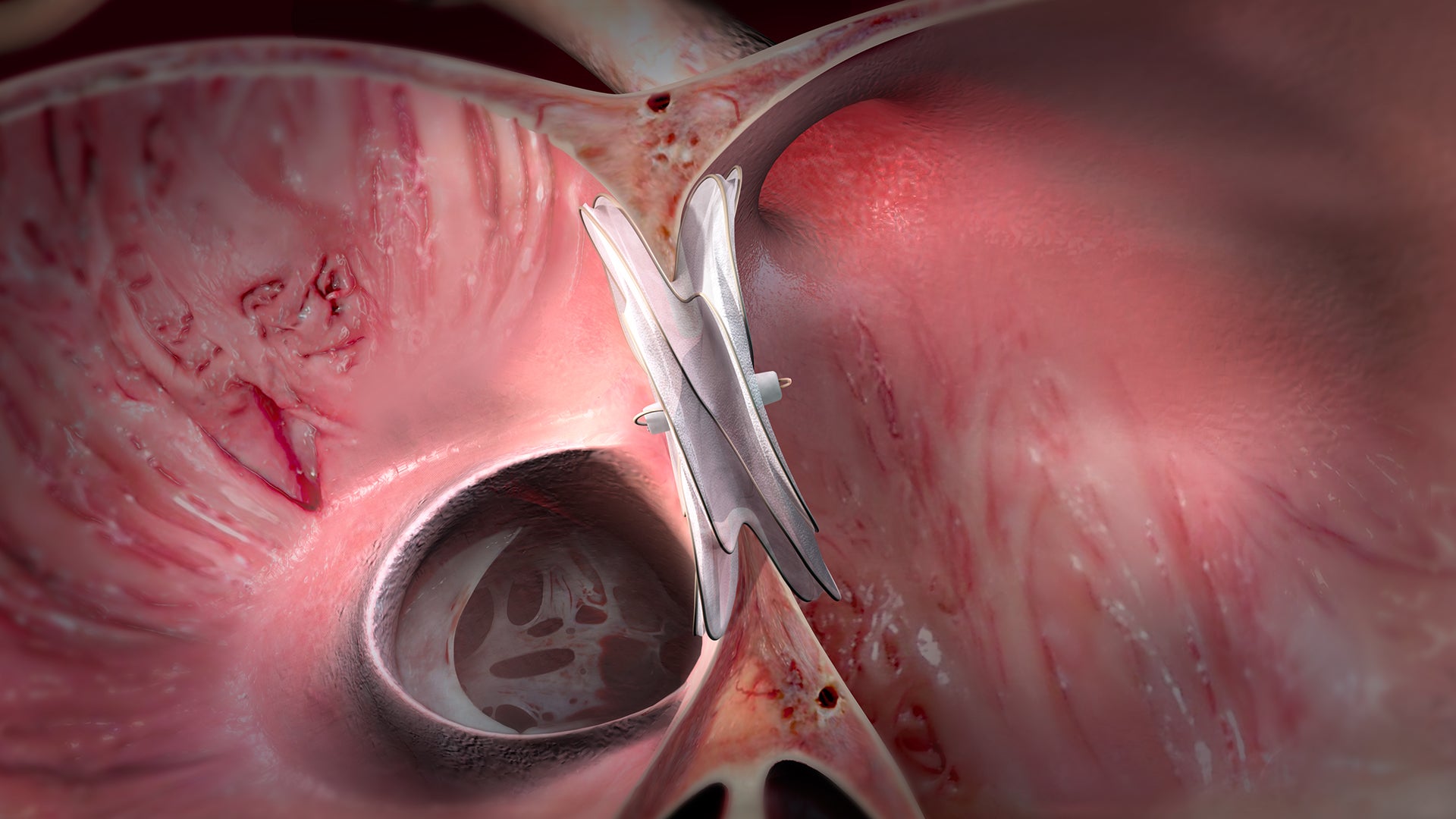
Provides value through improving clinical outcomes and lowering costs
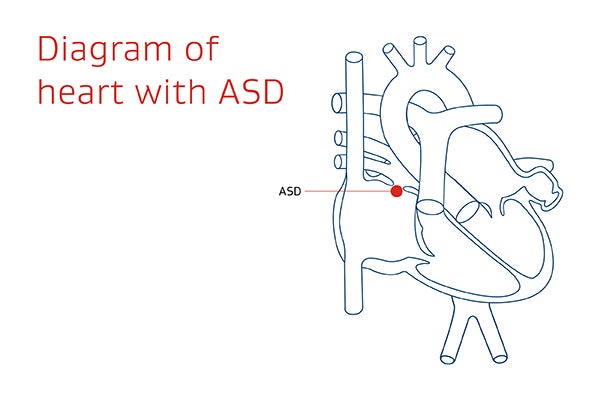
GORE® CARDIOFORM ASD Occluder is a device used for transcatheter closure of atrial septal defects (ASDs).*
An ASD is a hole in the wall between the heart’s upper chambers. It can cause extra blood to overfill the lungs and overwork the right side of the heart. Overtime, the right side of the heart can enlarge and/or weaken, causing potential problems such as: heart failure, arrhythmias, stroke and pulmonary hypertension.1
Fills and conforms to the defect for confident closure
- Soft and conformable construction designed to integrate with the natural structure of the atrial septum.
- Designed to minimize wall injury and allow tissue ingrowth for short- and long-term performance.
- Low device or procedure complications reported.2
100%
Closure success rate at six months†,2,3
Minimizing inventory and improving efficiencies with storing only five SKUs*
Only 5 SKUs
needed to treat patients with ASDs (8-35 mm)
The Future of Value Analysis
A Handbook for Health Care Professionals
Read perspectives from value analysis professionals who share their thoughts regarding the importance of effective collaboration, paradigm shifts with determining value, and the critical focus on the future of healthcare.

* See Instructions for Use at eifu.goremedical.com for full indications and sizing configurations.
† GORE® CARDIOFORM ASD Occluder effective closure rate results in device group subjects who received a study device. Effective closure defined as freedom from large shunt > 25 bubbles as detected by transthoracic echocardiography adjudicated by echo core lab.
- About congenital heart defects. Atrial septal defects. American Heart Association, Inc. website. September 24, 2020. https://www.heart.org/en/health-topics/congenital-heart-defects/about-congenital-heart-defects/atrial-septal-defect-asd
- GORE® CARDIOFORM ASD Occluder [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2020. MD177061.
- Sommer RJ, Love BA, Paolillo JA, et al; ASSURED Investigators. ASSURED clinical study: new GORE® CARDIOFORM ASD occluder for transcatheter closure of atrial septal defect. Catheterization & Cardiovascular Interventions 2020;95(7):1285-1295

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS/INTENDED USE: The GORE® CARDIOFORM ASD Occluder is a permanently implanted device indicated for the percutaneous, transcatheter closure of ostium secundum atrial septal defects (ASDs).
CONTRAINDICATIONS: The GORE® CARDIOFORM ASD Occluder is contraindicated for use in patients: Unable to take anti-platelet or anticoagulant medications such as aspirin, heparin, or warfarin; with anatomy where the GORE® CARDIOFORM ASD Occluder size or position would interfere with other intracardiac or intravascular structures, such as cardiac valves or pulmonary veins; with active endocarditis, or other infections producing bacteremia, or patients with known sepsis within one month of planned implantation, or any other infection that cannot be treated successfully prior to device placement; with known intracardiac thrombi.
