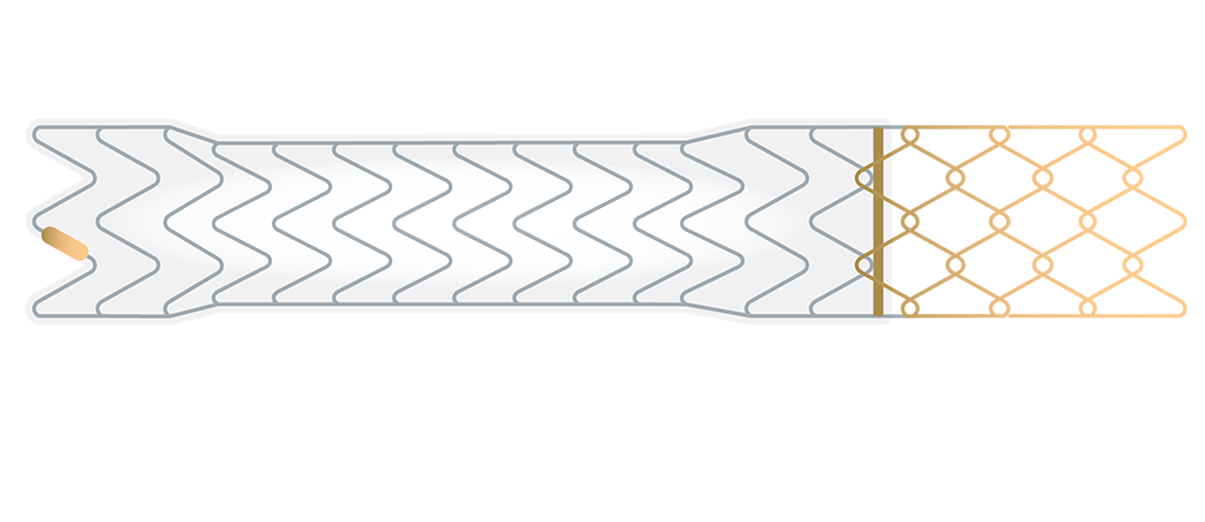
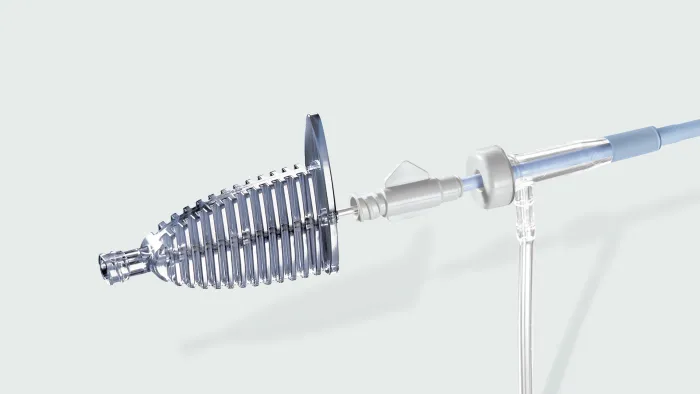
GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion
Lasting diameter control* and proven patency1
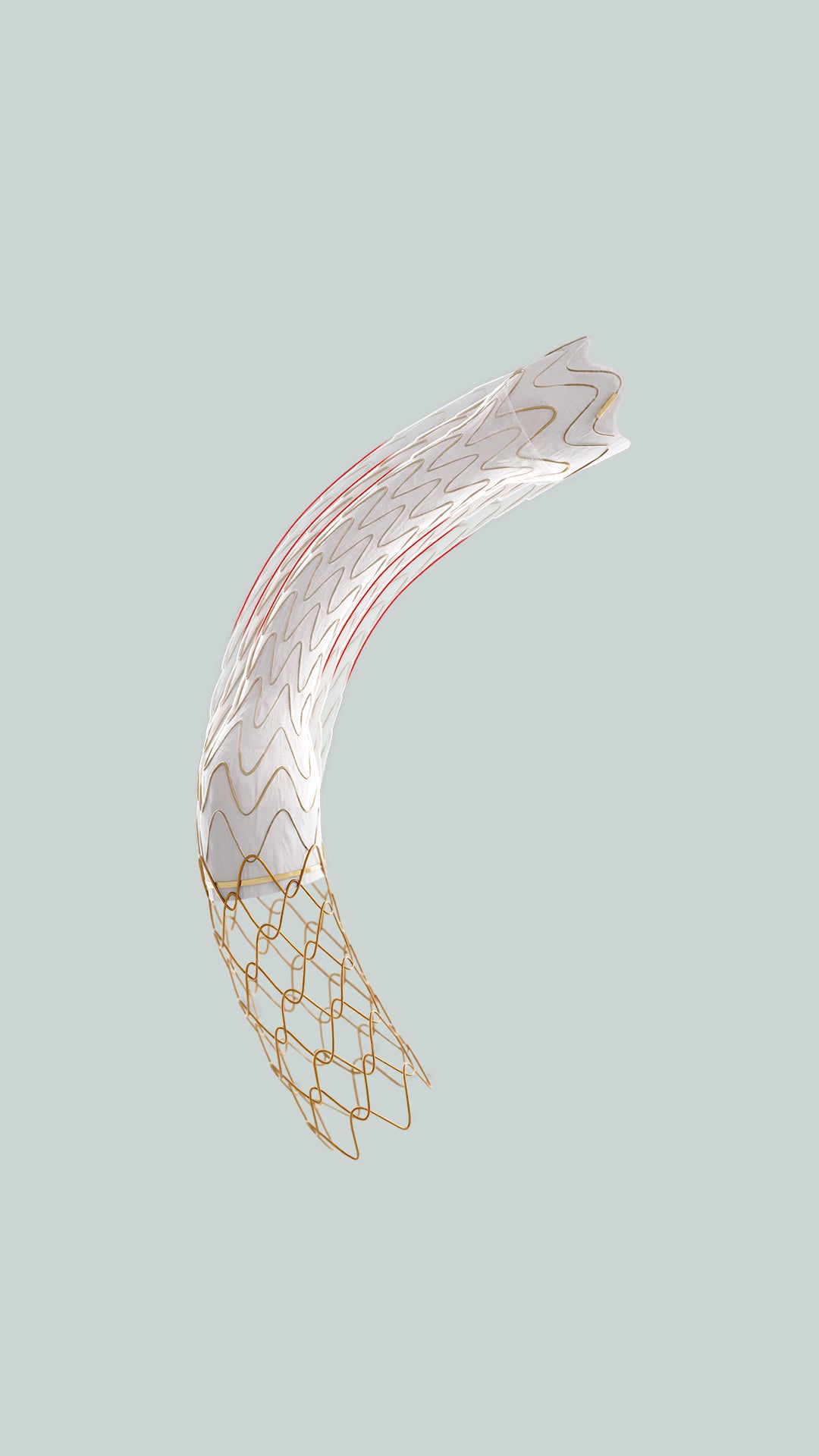
Purpose-built for TIPS
The only covered stent for TIPS with controlled expansion and 25 years of performance and safety data.†
Built for lasting performance
The VIATORR® Device allows you to select and maintain the diameter to reach the targeted portal pressure gradient. Choose a device feature to learn more.
ALTA-recommended for TIPS placement
The American Liver Therapeutic Approaches (ALTA) group recommends "the use of an ePTFE-lined stent graft with controlled expansion, which allows the operator to tailor the amount of portosystemic shunting based on the indication, target gradient and patient comorbidities.”2
Built for long-term results
76%
Two-year primary patency1
The VIATORR® Device was proven to maintain TIPS patency over time in a randomized controlled study (N = 80)1
Built for improved outcomes
Patients treated with the VIATORR® Device have a higher 1-year survival than those treated with non-TIPS therapy.2-4
Variceal bleeding outcomes
Early TIPS compared to drug therapy plus endoscopic band ligation (EBL) for variceal bleeding4
86%
1-year survival
following early TIPS procedure with the VIATORR® Device, compared to 61% 1-year survival following pharmacotherapy plus endoscopic band ligation (P < 0.001)‡,4
97%
1-year control of bleeding
following early TIPS procedure with the VIATORR® Device, compared to 50% 1-year survival following pharmacotherapy plus endoscopic band ligation (P < 0.001)§,4
No increase in the risk of hepatic encephalopathy
at 1 year, compared to pharmacotherapy plus endoscopic band ligation (P = 0.13)4
Refractory ascites outcomes
Early TIPS compared to large volume paracenteses (LVP) and albumin for refractory ascites3
Improved survival
TIPS with the VIATORR® Device demonstrated higher transplant-free survival (93%), compared to large volume paracenteses (LVP) and albumin infusion (52%) (P = .003)3
Fewer paracenteses
with 1 paracentesis procedure required to treat ascites following placement of the VIATORR® Device, compared to 10 procedures with paracentesis treatment alone3
No increase in the risk of hepatic encephalopathy
at 1 year, compared to large volume paracenteses (LVP) and albumin infusion (P = .868)3
Related to this product
* Based on benchtop data on file.
† Historical data from GORE® VIATORR® TIPS Endoprosthesis and GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion.
‡ For a combined group of patients with Child-Pugh C (CP-C) score ≤13 or Child-Pugh B with active bleeding (CP-B + AB) at diagnostic endoscopy.
§ One-year actuarial probability of remaining free of failure to control bleeding and of variceal rebleeding.
- Bureau C, Pagan JCG, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long term results of a randomized multicentre study. Liver International 2007;27(6):742-747.
- Boike JR, Thornburg BG, Asrani SK, et al. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clinical Gastroenterology and Hepatology. 2022;20(8):1636-1662.e36.
- Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152(1):157-163.
- García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. The New England Journal of Medicine 2010;362(25):2370-2379.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIATORR® TIPS Endoprosthesis is indicated for use in the de novo and revision treatment of portal hypertension and its complications such as variceal bleeding, gastropathy, refractory ascites, and / or hepatic hydrothorax.
INDICATIONS FOR USE IN CANADA: The GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion is indicated for the treatment of portal hypertension and its complications such as variceal bleeding and refractory ascites.
CONTRAINDICATIONS: There are no known contraindications for this device.