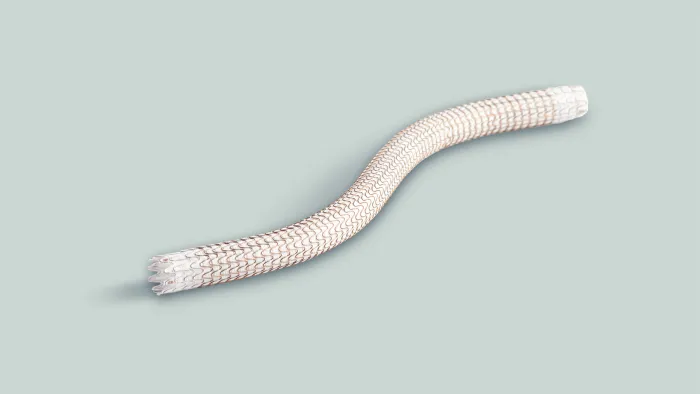
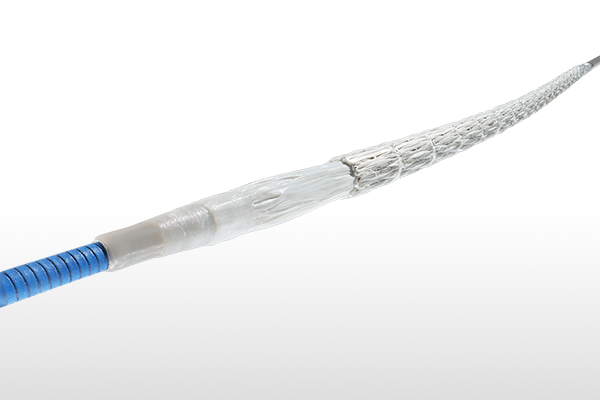
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
Designed to perform in challenging situations, the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft) offers proven procedural success and long-term outcomes* through flexibility, strength and accuracy to treat a wide range of clinical conditions.

Trusted performance. Unmatched versatility.†
The lower profile VBX Stent Graft is now available
Click below to see the product overview video:
Improvements to the device delivery system have enabled a 1 Fr profile reduction on the majority of sizes, now offering the most 6 Fr compatible configurations among balloon expandable stent grafts.1-4
Now 6 Fr compatible
GREATER VERSATILITY
UP TO 79 mm
stent length1-4
Advanced technology and unique design
Broadest offering of diameters and lengths2,4-6:
- The longest BX stent graft
- Broadest range of diameter adjustability in a single device†
- The most 6 Fr compatible configurations
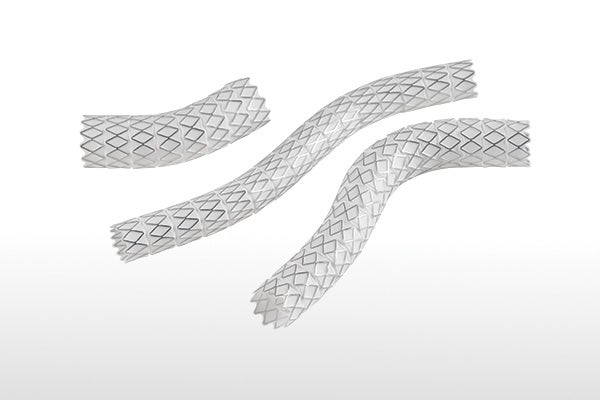
The only BX stent graft with stainless steel independent rings2,4-6:
- Enhances flexibility and conformability
- Minimizes foreshortening
- Provides high radial strength
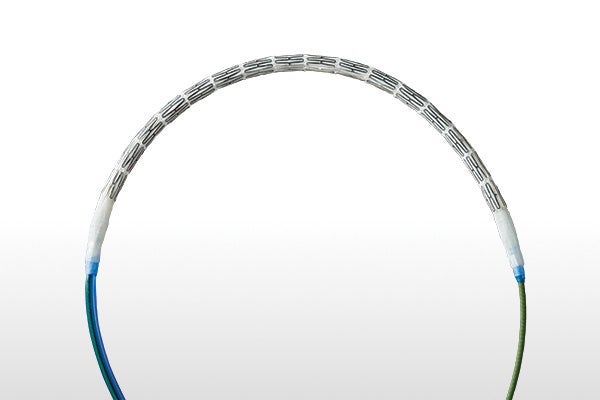
The only BX stent graft with a covered semi-compliant balloon2,4-6:
- Improves device retention on the catheter while tracking in tortous anatomy and tight angles
- Enables diameter customization
Proven leader in stent graft technology
- Twenty years of peripheral stent graft clinical experience
- Leverages the stent graft technology of the GORE® VIABAHN® Endoprosthesis
- Featuring Gore’s CBAS® Heparin Surface, the proven heparin bonding technology for lasting thromboresistance7
Animation - GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
Related to this product
* Based on prior clinical data. New evaluation of reduced profile delivery is underway.
† Across indications and configurations of covered stents.
ǂ As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
- GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis Instructions for Use (IFU). W. L. Gore & Associates, Inc. Accessed July 7, 2025. https://eifu.goremedical.com/
- LIFESTREAM® Balloon Expandable Vascular Covered Stent [Instructions for Use]. Tempe, AZ: Bard Peripheral Vascular, Inc; 2019. BAW1345700 Rev. 5 06/19
- iCast covered stent system [Instructions for Use]. Merrimack, NH: Atrium Medical Corporation; 2023. AW009603-EN Rev 11.
- Advanta V12 Balloon Expandable Covered Stent [Instructions for Use]. Merrimack, NH; Getinge AB. https://www.getinge.com/int/products/advanta-v12-balloon-expandable-covered-stent
- BeGraft Peripheral Plus Stent Graft System [Instructions for Use]. Hechingen, Germany: Bentley InnoMed GmbH. BeGraft Peripheral Stent Graft System [Instructions for Use]. Hechingen, Germany: Bentley InnoMed GmbH.
- iCover Peripheral balloon expandable PTFE covered stent system [Instructions for Use]. Barcelona, Spain. iVascular S.L.U.
- CBAS® Heparin Surface. W. L. Gore & Associates website. Accessed June 21, 2024. https://www.goremedical.com/en-emea/cbas-material-technology

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of:
- de novo or restenotic lesions found in iliac arteries, including lesions at the aortic bifurcation;
- de novo or restenotic lesions in the visceral arteries;
- isolated visceral, iliac, and subclavian artery aneurysms; or
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for use as a bridging stent in branched and fenestrated endovascular aortic aneurysm repair with the following§:
- Approved for use branched aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and polypropylene suture with branch diameters of 6/8 mm and lengths of 18/21 mm.
- Approved for use fenestrated aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and fenestrations with polypropylene suture ranging in diameter from 6–8 mm.
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
§ Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (BXB catalogue numbers).