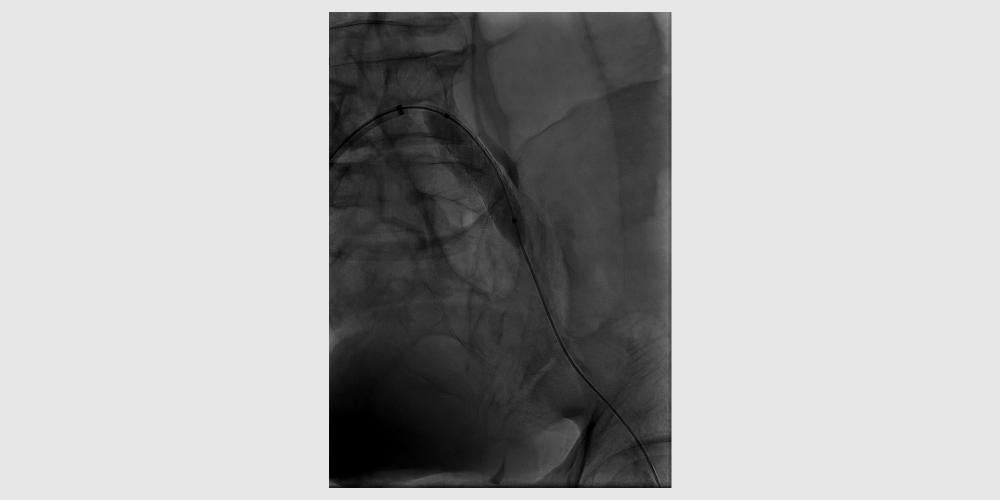
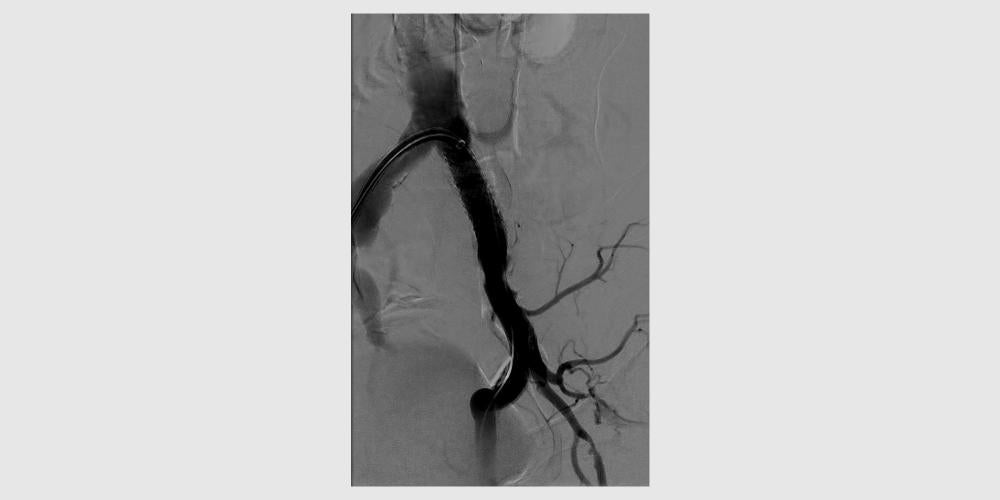
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis Case Studies: Treating a severely calcified lesion1
Patient presentation
- 63-year-old male; current smoker with history of diabetes, hypertension, hyperlipidemia, hypercholesterolemia
- No previous treatment for peripheral artery disease (PAD)
Clinical challenges
- Rutherford category 2 (moderate claudication)
- Severely calcified 80% stenosis in left common iliac
- Bismuth J, Gray BH, Holden A, Metzger C, Panneton J; VBX FLEX Study Investigators. Pivotal study of a next-generation balloon-expandable stent-graft for treatment of iliac occlusive disease. Journal of Endovascular Therapy 2017;24(5):629-637. http://journals.sagepub.com/doi/full/10.1177/1526602817720463
Procedural outcomes based on usage of legacy GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXA catalogue numbers).
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries, including lesions at the aortic bifurcation;
- de novo or restenotic lesions in the visceral arteries;
- isolated visceral, iliac, and subclavian artery aneurysms; or
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for use as a bridging stent in branched and fenestrated endovascular aortic aneurysm repair with the following:
- Approved for use branched aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and polypropylene suture with branch diameters of 6/8 mm and lengths of 18/21 mm.
- Approved for use fenestrated aortic endovascular grafts constructed of polyester fabric, stainless steel stents, and fenestrations with polypropylene suture ranging in diameter from 6–8 mm.
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis with Reduced Profile:
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries, including lesions at the aortic bifurcation;
- de novo or restenotic lesions in the visceral arteries;
- isolated visceral, iliac, and subclavian artery aneurysms; or
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries).
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.