How the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* changed the natural history of a rapidly failing arteriovenous (AV) access circuit
A case study using the VIABAHN® Device
Challenge
- Severe stenosis across the elbow of a 78-year-old patient.
- A rapidly failing forearm loop graft that thrombosed three times over a 40-day period.
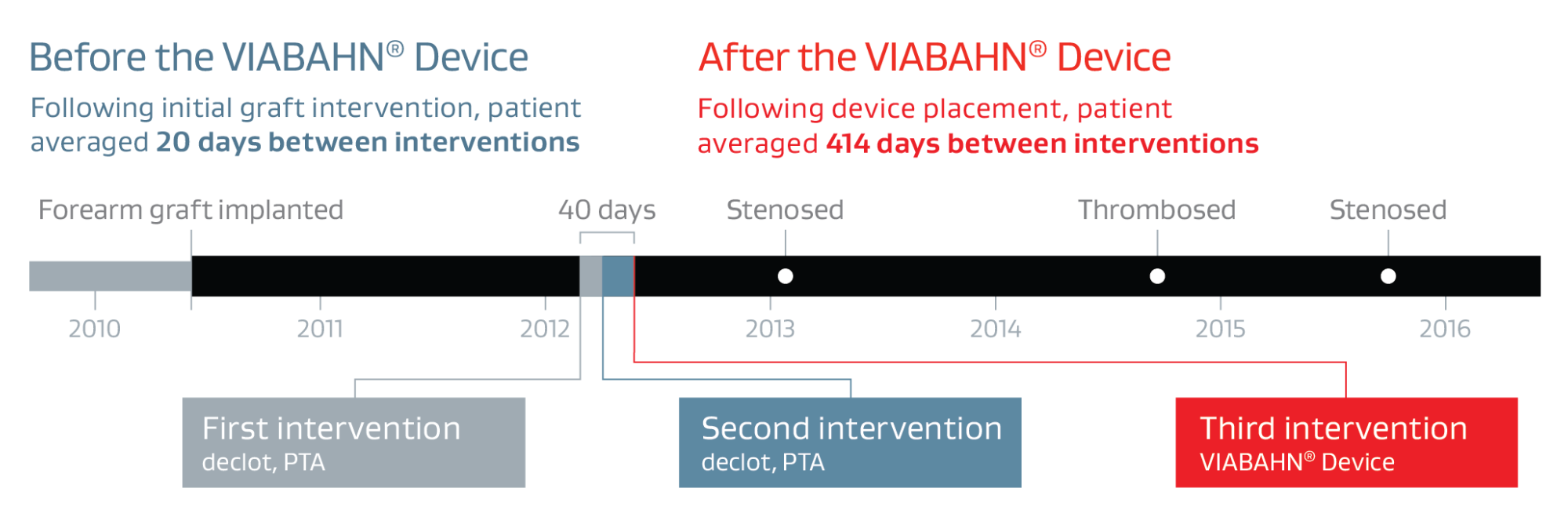
- The graft worked well for nearly two years before the first of the three thrombosis events.
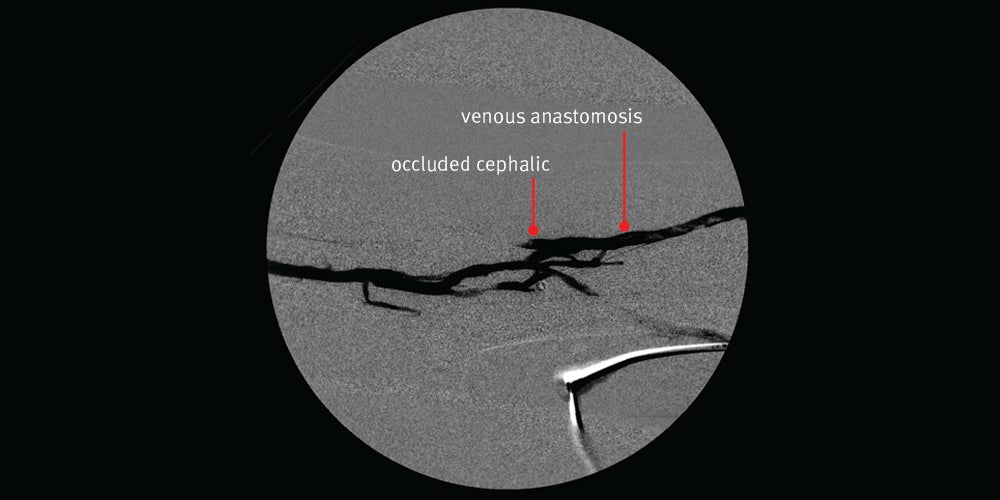
First intervention — Declot percutaneous transluminal angioplasty (PTA) was chosen to treat the thrombosed graft
Declot PTA procedure:
- The thrombosed graft was secondary to a severe venous anastomotic stenosis.
- The circuit had been working well for nearly two years.
Treatment rationale:
- Give the lesion an opportunity to “declare its natural history”
- KDOQI 6.7 – treatment of thrombosis and associated stenosis
- 6.7.5 stenoses should be corrected by using angioplasty or surgical revision
Result:
- Nice angiographic result initially with restored flow however, the patient returned 12 days later with a clotted graft
Images courtesy of Minneapolis Vascular Physicians. Used with permission.
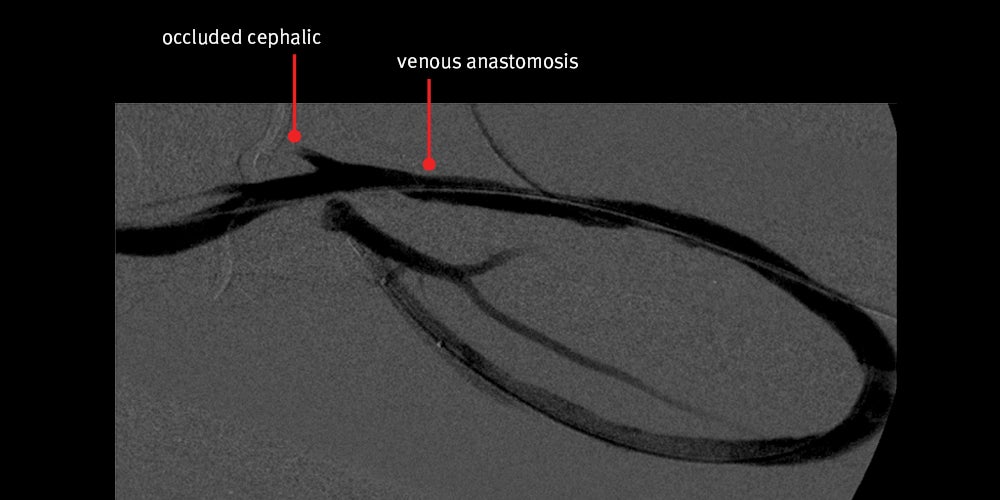
Second intervention — Declot PTA was chosen again to treat the thrombosed graft.
Declot PTA procedure:
- Only 12 days passed after the first intervention.
- Patient again presented with thrombosed graft secondary to severe venous anastomotic stenosis.
Treatment rationale:
- Continue to allow the lesion an opportunity to “declare its natural history”
- KDOQI 6.6 – If angioplasty of the same lesion is required more than two times within a three-month period, the patient should be considered for surgical revision if the patient is still a good surgical candidate
- Still has not exceeded the KDOQI recommendation
Result:
- Again, nice angiographic result achieved initially with restored flow however, the patient returned 28 days later with a clotted graft
Images courtesy of Minneapolis Vascular Physicians. Used with permission.
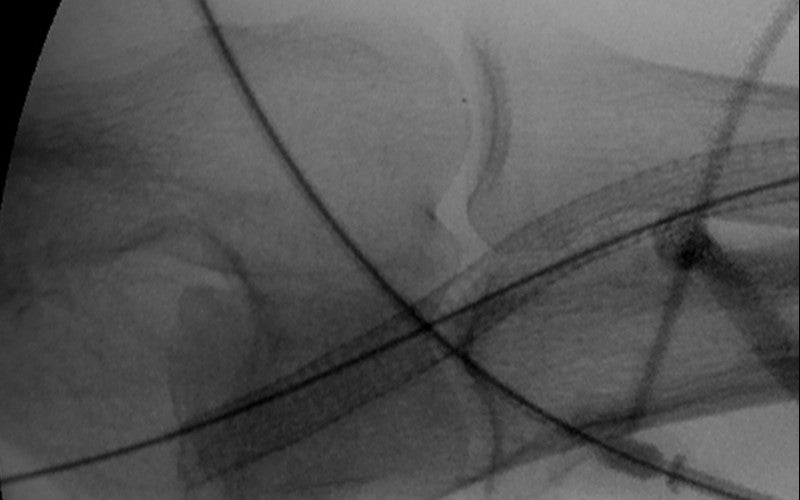
Third intervention —VIABAHN® Device placed across the elbow to treat recurrent severe venous anastomotic stenosis
The VIABAHN® Device procedure
- Placed the VIABAHN® Device across the elbow instead of abandoning circuit.
Treatment rationale:
- Exceeded KDOQI 6.6 recommendation – If angioplasty of the same lesion is required more than two times within a three-month period, the patient should be considered for surgical revision if the patient is still a good surgical candidate
- Accurate deployment of the VIABAHN® Device allows for treatment of stenosis across elbow while saving upper arm real estate
Results:
- Nice angiographic result. Flow was restored.
- Accurate deployment left open the possibility of upper arm fistula
- Long term, the VIABAHN® Device broke the clotting cycle of this graft
- The patient returned for three interventions (two PTA of stenosis and one declot) from May 2012 to January 2016, with an average of 414 days between interventions
Images courtesy of Minneapolis Vascular Physicians. Used with permission.
Case Takeaways
Placing the VIABAHN® Device across the elbow extended the life of the graft without sacrificing the opportunity for an upper arm access.
* As used by Gore, PROPATEN Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of Healthcare Providers. Healthcare Providers remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.