Treating a thrombosed arteriovenous (AV) access graft with the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*
A case study using the VIABAHN® Device
Challenge
- 77-year-old female with end-stage renal disease on hemodialysis via a right upper extremity axillary artery to axillary vein ePTFE loop graft
- Presented after unsuccessful attempt at dialysis due to an occluded graft
- Relevant patient history:
- Access was created four months prior to presentation
- Venogram performed two months prior to presentation for pulsatility and recirculation demonstrated venous anastomotic stenosis, for which drug-eluting balloon venoplasty was performed
Procedure
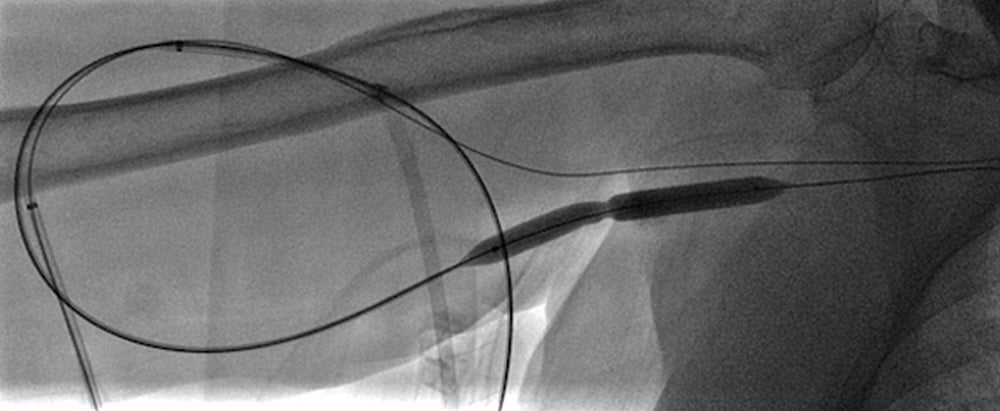
- Initial ultrasound images demonstrated the length of the graft to be occluded. Antegrade and retrograde access was obtained.
- Pharmacological thrombolysis was performed with 4 mg tPA followed by balloon
- Contrast was injected via the antegrade sheath, demonstrating persistent moderate stenosis
- Given short-term recurrence and graft occlusion, an 8 mm x 7.5 cm VIABAHN® Device was deployed to treat the recurrent venous anastomotic stenosis
Result
- Completion AV graft venogram demonstrated the graft and venous outflow stent to be widely patent
- A brisk thrill was palpated
- Patient had successful hemodialysis immediately following the procedure and dialysis access circuit remains patent
Case Takeaways
- The VIABAHN® Device remains an excellent option for percutaneous revision of venous anastomotic stenosis in patients with a prosthetic hemodialysis graft
- In this patient with short-term recurrent stenosis after drug-eluting balloon venoplasty, use of the VIABAHN® Device aided in re-establishing access circuit patency
Related case study
*As used by Gore, PROPATEN Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use (IFU) or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.