Treating claudication and rest pain due to chronic total occlusion of the SFA
A case study using the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*
Procedure
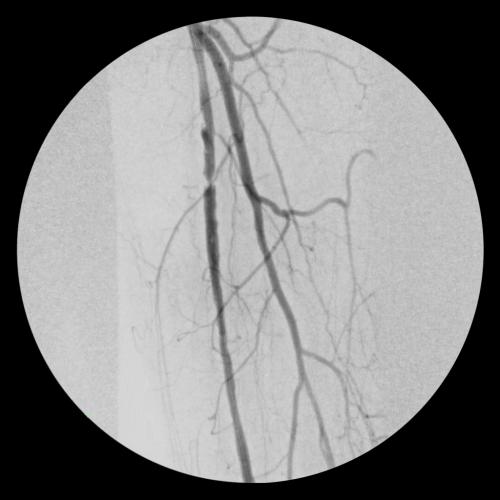
- Obtained percutaneous access into the right femoral artery with ultrasound guidance. Then performed an angiogram of the left lower extremity.
- Crossed SFA chronic total occlusion (CTO) with 0.035” TERUMO® RADIOFOCUS® GLIDEWIRE® ADVANTAGE and 0.035” TERUMO® NAVICROSS® Support Catheter
- Atherectomy completed with CARDIOVASCULAR SYSTEMS DIAMONDBACK 360® Peripheral Orbital Atherectomy System 2.0 Solid Crown
- Followed by angioplasty with a 5 mm percutaneous transluminal angioplasty (PTA) balloon
- Deployed two 6 mm x 15 cm VIABAHN® Devices in the SFA and post-dilated with 6 mm PTA balloon
Result
- Completion angiogram showed excellent flow of the left lower extremity. Immediately post-op, complete resolution of left sided severe claudication.
- At follow-up office visit 2.5 weeks post-op, the patient presented with complete resolution of claudication and rest pain. Patient had palpable tibial pulses bilaterally
Case takeaways
As demonstrated in this case, the VIABAHN® Device offers excellent patency in the treatment of long SFA total occlusions and should be considered first-line treatment for complex SFA disease
The VIABAHN® Device has demonstrated durability and long-term patency in treatment of complex SFA disease with
97% three-year secondary patency
(27 cm average lesion length, 93% CTOs)1
Related case study
* As used by Gore, PROPATEN Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
- Böhme T, Noory E, Brechtel K, et al. Heparin-bonded stent-graft for the treatment of TASC II C and D femoropopliteal lesions: 36-month results of the Viabahn 25 cm trial. Journal of Endovascular Therapy. 2021;28(2) 222-228. https://journals.sagepub.com/doi/pdf/10.1177/1526602820965965
This content is for informational purposes only, is not advice or a guarantee of outcome. It is not a substitute for professional medical advice, diagnosis or treatment. Individual results and/or treatment may vary based upon the circumstances, the patient’s specific situation, and the healthcare provider’s medical judgment.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.