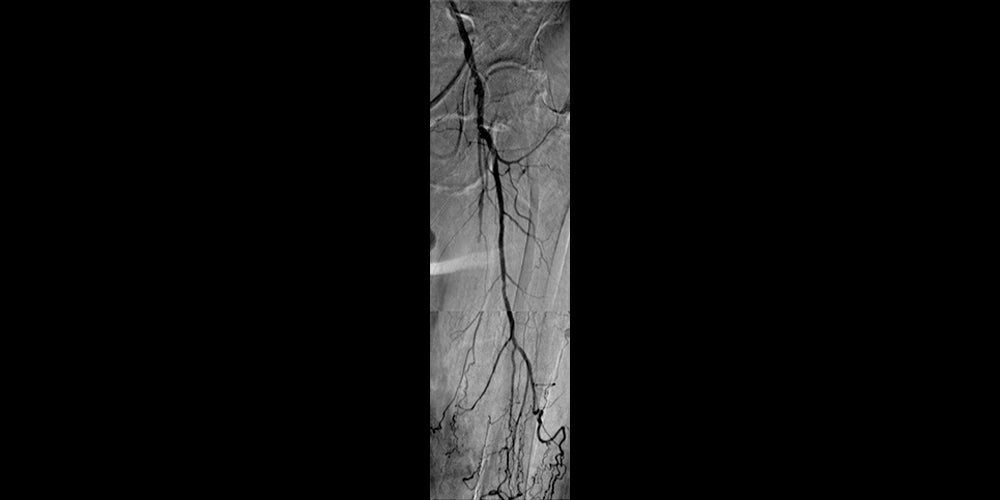
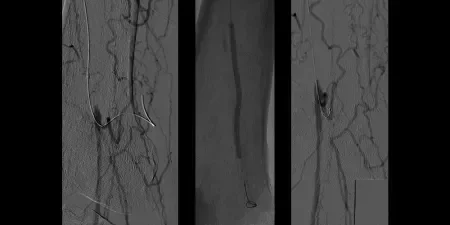
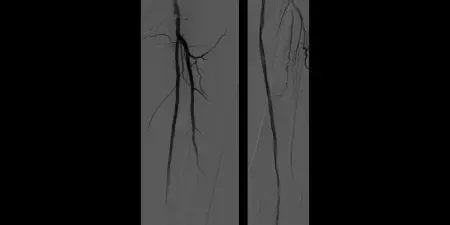
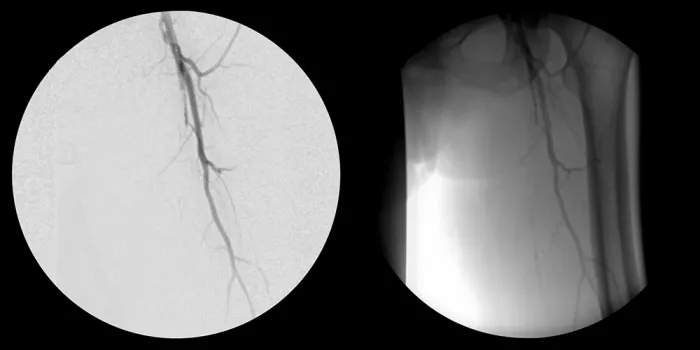
Non-healing ulcer with superficial femoral artery (SFA) disease
A case study using the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*
Challenge
- 72-year-old male with non-healing left foot ulceration (Rutherford 5) and leg edema
- Relevant patient history:
- Diabetes with peripheral neuropathy
- Congestive heart failure
- Prior interventions:
- February 2009 – Left femoral to below-knee bypass with right saphenous vein (claudication)
- September 2010 – Vein bypass thrombosed, with unsuccessful attempt to treat the graft at outside institution. Developed non-healing ulcer on top of left foot. Calcified ankle-brachial indexes (ABIs), with toe pressure of 20 mmHg on left and 34 mmHg on right. No palpable pulses below left femoral level.
- October 2011 – Left SFA treatment
Result
- Maintained one-vessel runoff
- The patient went home after three hours with palpable dorsalis pedis pulse, ABI of 1.0
- Long-term anticoagulation / antiplatelet regimen: Warfarin and ASPIRIN (acetylsalicylic acid) Surveillance with duplex ultrasound within one month then at six months
- No recurrence of ulceration or need for other interventions and no amputations
- The patient died 11 months later (in September 2012) of respiratory failure associated with his congestive heart failure
Case takeaways
Successful recanalization of SFA CTO in critical limb ischemia (CLI) patient
- Good inline flow through the SFA with preservation of tibial runoff
- Healing of ulcer and limb preservation in CLI patient
- Consider duplex ultrasound at frequent intervals through the first year, especially when runoff is limited
Related case study
CORDIS® and OUTBACK® are trademarks of Cordis Corp.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use (IFU) or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.
* As used by Gore, Heparin Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is indicated for the treatment of:
- de novo or restenotic lesions in the iliac arteries
- de novo or restenotic lesions in the superficial femoral artery and proximal popliteal artery
- in-stent restenotic lesions in the superficial femoral artery and proximal popliteal artery
- stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts and in the venous outflow of dialysis access circuits, including the central veins
- popliteal artery aneurysms and isolated visceral artery aneurysms
- traumatic or iatrogenic vessel injuries in arteries that are located in the chest cavity, abdominal cavity, or pelvis (except for aorta, coronary, innominate, carotid, vertebral, and pulmonary arteries)
CONTRAINDICATIONS:
- Non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system.
- Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.