PRESERVATION MATTERS — A.L.W.A.Y.S
GORE® EXCLUDER® Iliac Branch Endoprosthesis
Sustaining quality of life
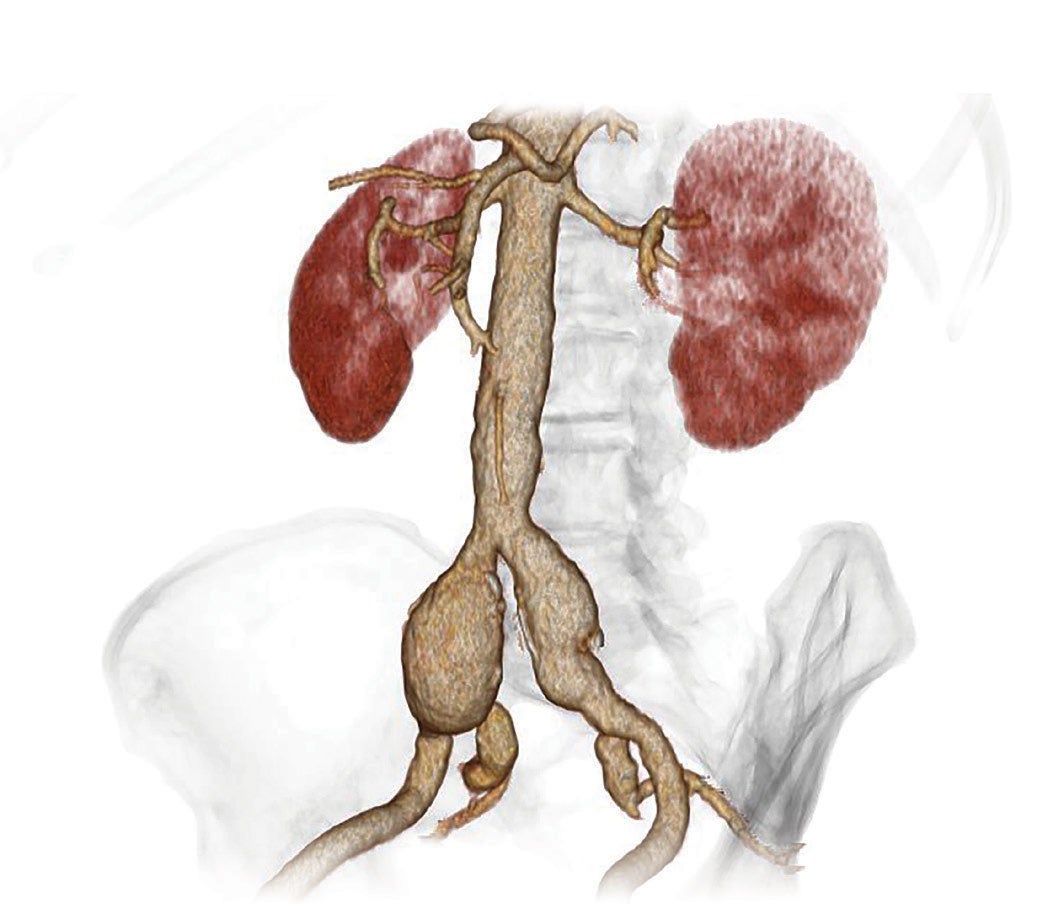
Hypogastric artery preservation is the recommended treatment1,2 to sustain quality of life. When determining which preservation method is best, A. L. W. A. Y. S.3,4 consider each patient’s:
Hypogastric artery preservation: A.L.W.A.Y.S. provides a simple way to ensure key considerations are included in your decision.
The information provided is intended to be general guidance based on current medical practices in the field. The steps described here may not be complete and are not intended to be a replacement for the Instructions for Use (IFU) or the education, training and professional judgment of health care providers (HCPs). Licensed HCPs remain responsible for making decisions about patient care and the use of medical technologies.
A story of preservation
A patient and his physician talk about the treatment options and considerations specific to his active lifestyle.
The content of this video is intended for informational use only and is not intended to be a substitute for professional medical diagnosis, care or therapy. The patient story and outcomes presented here are specific to the particular patient. Individual responses to medical care may vary from patient to patient.
- Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of Vascular Surgery 2018;67(1):2-77.e2.
- Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. European Journal of Vascular & Endovascular Surgery 2011;41(Supplement 1):S1-S58.
- Million A, Milner R, Oderich GS, Schneider DB, Spark I, Verzini F. Expert discussion: when to preserve the hypogastric artery. Endovascular Today 2017;16(8)Supplement:6-11.
- Schneider DB, Milner R, Heyligers JMM, Chakfé N, Matsumura J. Outcomes of the GORE Iliac Branch Endoprosthesis in clinical trial and real-world registry settings. Journal of Vascular Surgery 2019;69(2):367-377.e1.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: Iliac Branch and Internal Iliac Components. The GORE® EXCLUDER® Iliac Branch Endoprosthesis (IBE) is intended to be used with the GORE® EXCLUDER® AAA Endoprosthesis or the GORE® EXCLUDER® Conformable AAA Endoprosthesis to isolate the common iliac artery from systemic blood flow and preserve blood flow in the external iliac and internal iliac arteries in patients with a common iliac or aortoiliac aneurysm, who have appropriate anatomy, including: adequate iliac/ femoral access; minimum common iliac diameter of 17 mm at the proximal implantation zone of the IBE; external Iliac artery treatment diameter range of 6.5–25 mm and seal zone length of at least 10 mm; internal iliac artery treatment diameter range of 6.5–13.5 mm and seal zone length of at least 10 mm; adequate length from the lowest major renal artery to the internal iliac artery to accommodate the total endoprosthesis length, calculated by adding the minimum lengths of required components, taking into account appropriate overlaps between components. GORE® EXCLUDER® AAA Endoprosthesis Components used in conjunction with GORE® EXCLUDER® Iliac Branch Endoprosthesis. Trunk-Ipsilateral Leg Component. The Trunk-Ipsilateral Leg is intended to provide proximal seal and fixation for the endovascular repair of the aneurysm. For more information on the Trunk-Ipsilateral Leg Component indications for use and deployment, see the GORE® EXCLUDER® AAA Endoprosthesis or the GORE® EXCLUDER® Conformable Endoprosthesis Instructions for Use. Contralateral Leg Endoprosthesis Component. The Contralateral Leg Endoprosthesis is intended to bridge the GORE® EXCLUDER® Device Trunk-Ipsilateral Component to the GORE® EXCLUDER® Iliac Branch Endoprosthesis following deployment of the GORE® EXCLUDER® Iliac Branch Endoprosthesis. Additionally, the Contralateral Leg Endoprosthesis is intended to be used for distal extension of the Iliac Branch Component in the external iliac artery. The Iliac Branch Component can treat external iliac artery diameters up to 13.5 mm. This ability to extend the Iliac Branch Component distally with any Contralateral Leg Endoprosthesis expands the external iliac artery treatment range up to 25 mm. For more information on the Trunk-Ipsilateral Leg and Contralateral Leg Endoprosthesis Component indications for use and deployment, see the GORE® EXCLUDER® AAA Endoprosthesis Instructions for Use. Aortic Extender and Iliac Extender Components. The Aortic and Iliac Extender Components can be used after deployment of the GORE® EXCLUDER® Iliac Branch and GORE® EXCLUDER® AAA Endoprostheses. These extensions are used when additional length and/or sealing for aneurysmal exclusion is desired.
CONTRAINDICATIONS: The GORE® EXCLUDER® Iliac Branch Endoprosthesis is contraindicated in: patients with known sensitivities or allergies to the device materials. All components of the GORE® EXCLUDER® Iliac Branch Endoprosthesis and the GORE® EXCLUDER® AAA Endoprosthesis contain ePTFE, FEP, nitinol (nickel-titanium alloy) and gold; patients with a systemic infection who may be at increased risk of endovascular graft infection.