Catalogue numbers for GORE® CARDIOFORM Septal Occluder

Device Specifications
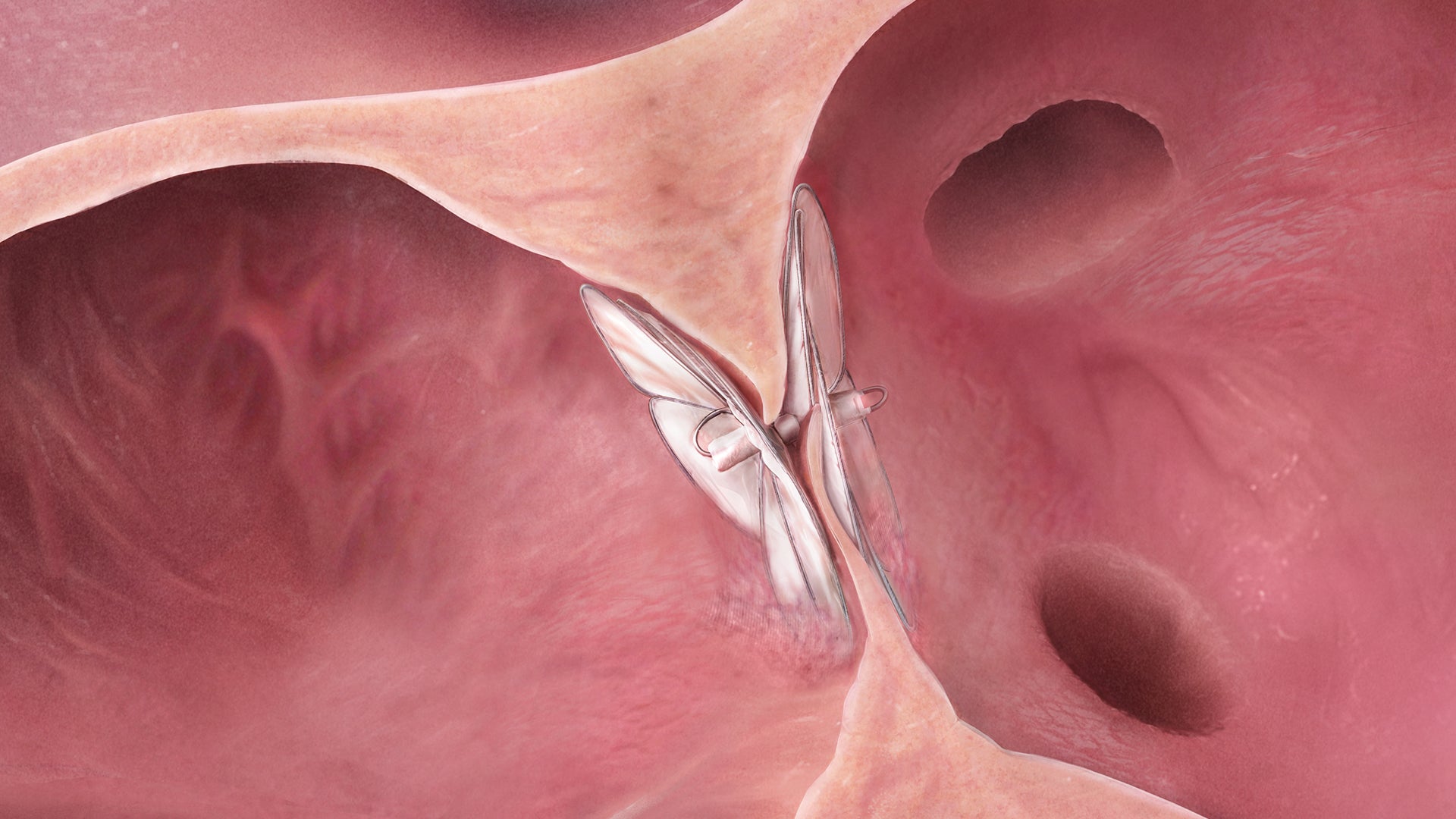
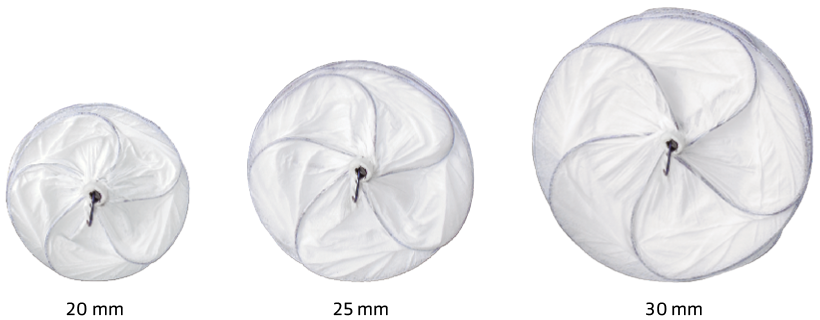
With the conformable design of the GORE® CARDIOFORM Septal Occluder, three devices cover patent foramen ovales (PFOs) and ostium secundum atrial septal defects (ASDs) up to 17 mm.*
| Catalogue Number | Device Sizes | Maximum Recommended Defect Size Measured with Stop Flow Balloon Sizing | Catheter Size without Guidewire† |
|---|---|---|---|
| GSXE0020 | 20 mm | 11 mm | 10 Fr |
| GSXE0025 | 25 mm | 14 mm | 10 Fr |
| GSXE0030 | 30 mm | 17 mm | 10 Fr |
Sizing, availability and pricing varies by country.
* Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
† Recommendation for sheath size is 2 Fr larger when used with a wire.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN EUROPE: The GORE® CARDIOFORM Septal Occluder is a permanently implanted device indicated for the percutaneous, transcatheter closure of atrial septal defects (ASDs), such as ostium secundum and patent foramen ovale.
CONTRAINDICATIONS: The GORE® CARDIOFORM Septal Occluder is contraindicated for use in patients:
- Unable to take anti-platelet or anticoagulant medications such as aspirin, heparin, or warfarin.
- With anatomy where the GORE® CARDIOFORM Septal Occluder size or position would interfere with other intracardiac or intravascular structures, such as cardiac valves or pulmonary veins.
- With active endocarditis, or other infections producing bacteremia, or patients with known sepsis within one month of planned implantation, or any other infection that cannot be treated successfully prior to device placement.
- With known intracardiac thrombi.